Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Nature Communications | Chinese Medical Team Innovates CAR-T Therapy to Bring New Hope for AML Treatment
Nature Communications | Chinese Medical Team Innovates CAR-T Therapy to Bring New Hope for AML Treatment

AML
In the field of hematological malignancies, the treatment of acute myeloid leukemia (AML) has long been a challenge in the medical community. Particularly for relapsed or refractory patients, the limitations of traditional treatments have increased the urgent need for new therapies. With the advancements in immunotherapy technology, the continuous updating of CAR-T cell therapy has brought new possibilities for the treatment of AML.
Recently, a study titled “CAR-T Cells with C-JUN Overexpression in Acute Myeloid Leukemia: Preclinical Features and Phase I Trial” was published in the journal Nature Communications. This study comprehensively explores the potential optimization mechanisms of CAR-T therapy in AML and was jointly completed by doctors and professors from three top hospitals in China.
During an interview, the Chinese medical team stated that in designing this study, they focused on the major obstacles in CAR-T cell therapy for AML. Although CD33, a highly expressed target, has been extensively explored in multiple studies, safety and efficacy remain challenges. The study found that high expression of CD155 affects the ERK signaling pathway, thereby hindering the effective expansion of CD33. To address this challenge, the research team ultimately selected the overexpression of C-JUN to enhance the exhaustion resistance of CAR-T cells by screening ERK pathway genes. Preliminary results from the clinical trial showed that this overexpressed CD33 CAR-T cell exhibited significant advantages in antitumor function.
Despite the progress, the challenges in CAR-T therapy for AML treatment were also emphasized, including the balance between high efficacy and low toxicity, the expansion of CAR-T cells in myeloid leukemia cells, and the affinity of antibody binding. Subsequent research will focus more on addressing these key issues to enable more patients to benefit from CAR-T therapy.
The Chinese clinical trials revealed some key findings of CAR-T therapy, such as the effectiveness of the bridging transplant strategy and the remarkable efficacy of CD33 CAR-T cells derived from transplant donors. These studies not only provide new insights into the application of CAR-T therapy in AML but also offer references for the optimization of therapies targeting other antigens.
The Chinese medical team will continue to conduct in-depth research around the three core issues of toxicity, expansion, and antigen binding, aiming to overcome challenges and enable more patients to benefit from the breakthrough results of CAR-T cell therapy.
����To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#CAR_TCellTherapy #AcuteMyeloidLeukemia #AMLResearch #ImmunotherapyAdvancements #ChineseMedicalInnovation #CancerTreatment #MedicalBreakthrough #NatureCommunications #OncologyResearch #HematologicalMalignancies
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Overcoming Challenges and Embracing New Life! – Breakthrough Progress of Eque-cel in the Treatment of Refractory Multiple Myeloma
### Overcoming Challenges and Embracing New Life! – Breakthrough Progress of Eque-cel in the Treatment of Refractory Multiple Myeloma

Multiple Myeloma
In the treatment of multiple myeloma (MM), how do we find new breakthroughs for patients who have not achieved complete remission (CR) after multiple rounds of chemotherapy? Research by Chinese medical professors has provided an exciting answer: Eque-cel (BCMA CAR-T therapy).
**Patient Background:**
This 58-year-old female patient was initially admitted to the hospital due to numbness and pain in both lower limbs and was eventually diagnosed with multiple myeloma. Despite receiving various treatment regimens, including VRD and SVPD, the results were unsatisfactory, and complete remission was not achieved. Faced with refractory characteristics, the doctors decided to try a more innovative treatment plan—CAR-T cell therapy.
**Treatment Process:**
In September 2023, the patient began peripheral blood mononuclear cell collection, followed by bridging therapy, and in November 2023, she received the Eque-cel infusion. Remarkably, just one month later, the patient achieved hematologic complete remission (CR) with minimal residual disease (MRD) negativity. After six months of follow-up, the patient maintained this excellent therapeutic effect.
**Professor’s Insights:**
Chinese medical professors pointed out that the advent of Eque-cel has brought new hope to refractory MM patients. The drug demonstrated significant efficacy in the FUMANBA-1 study: the overall response rate was as high as 98.9%, with 82.4% achieving complete remission, and 97.8% of patients achieving MRD negativity. The 12-month sustained MRD negativity rate reached 81.7%, and the PFS rate was 85.5%.
This outstanding result proves the significant advantage of Eque-cel in improving the depth of remission for MM patients, bringing hope for long-term survival to many refractory patients.
**Future Outlook:**
As the application and research of Eque-cel continue, we look forward to it providing better treatment options and survival opportunities for more MM patients. This new treatment plan is bringing a ray of hope to this stubborn disease and providing valuable experience for clinical experts worldwide.
**Stay Tuned:**
We will continue to follow the latest developments and research progress of Eque-cel, looking forward to its greater role globally, bringing hope and blessings to more patients.

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp:+8613717959070
Email: doctor.huang@globecancer.com
#MultipleMyeloma #CARTTherapy #MedicalBreakthrough #EqueCel #CancerTreatment #MedicalResearch #Hematology #PatientCare #OncologyInnovation #HopeForPatients #BCMA #CART #MM #RRMM
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Blood Cancer Solution Including leukemia, lymphoma, multiple myeloma, and others.
 Blood Cancer Solution
Blood Cancer Solution
 Including leukemia, lymphoma, multiple myeloma, and others.
Including leukemia, lymphoma, multiple myeloma, and others.

Blood Cancer
#leukemia #lymphoma #multiplemyeloma
#Hematologic malignancies are a group of malignant diseases originating from hematopoietic cells, often affecting the bone marrow, blood, and various organs and tissues throughout the body. Common types of hematologic malignancies include leukemia, myelodysplastic syndromes, lymphomas, multiple myeloma, and myeloproliferative neoplasms.
The causes of these diseases are complex, involving genetic mutations, immune abnormalities, radiation exposure, contact with harmful chemicals, infections, and hereditary factors. Additionally, poor lifestyle habits, high levels of stress, and environmental factors can also increase the risk of developing these conditions.
With an aging population and advancements in medical technology, the incidence of hematologic malignancies has been rising globally. In China, the incidence and mortality rates of leukemia and lymphoma are now among the top ranks of all malignancies.
However, hematologic malignancies are not incurable. In recent years, the treatment methods for these diseases have seen significant progress. From traditional combination chemotherapy and radiotherapy to hematopoietic stem cell transplantation, monoclonal antibody therapy, antibody-drug conjugates, small molecule targeted therapies, and the latest immunotherapies, treatment options have become increasingly diverse and precise.
Combination chemotherapy remains a primary treatment for many hematologic malignancies, despite its significant side effects. The efficacy of these treatments cannot be ignored. Modern chemotherapy regimens are continually being refined, including the incorporation of new cytotoxic drugs and targeted therapies, as well as the use of monoclonal antibodies. Additionally, the appropriate use of antiemetics, hematopoietic growth factors, and anti-infective agents helps to mitigate adverse effects.
Hematopoietic stem cell transplantation continues to be one of the most effective treatments for certain hematologic malignancies. The development of this treatment in China has been rapid, with 170 registered transplant centers by 2020.
Monoclonal antibodies, often referred to as “biological missiles,” have a high degree of specificity and single biological activity. They have revolutionized the treatment of hematologic malignancies. Antibody-drug conjugates (ADCs) utilize monoclonal antibodies to accurately identify tumor cell markers, guiding the delivery of chemotherapy drugs for targeted treatment.
Small molecule targeted therapies work by interfering with specific genes or proteins to inhibit tumor cell growth and proliferation. Gleevec, the first small molecule targeted therapy, increased the five-year survival rate for chronic myeloid leukemia (CML) patients from 30% to 89%, marking a breakthrough in cancer treatment. Today, there are numerous small molecule targeted drugs available for the treatment of hematologic malignancies, including BCR-ABL inhibitors, BTK inhibitors, BCL-2 inhibitors, PI3K inhibitors, and XPO1 inhibitors, with many more drugs currently in clinical trials expected to become available soon.
Immunotherapy includes immune checkpoint inhibitors (such as PD-1/L1), cancer vaccines, cellular immunotherapies (such as #CART), and nonspecific immunomodulatory treatments. #CARTtherapy, in particular, has gained widespread attention as an emerging curative treatment. This approach involves extracting a patient’s T cells, modifying them outside the body to specifically recognize and attack tumor cells, and then reinfusing the modified T cells into the patient. This therapy has been successfully applied to various hematologic malignancies, including acute lymphoblastic leukemia, lymphomas, and multiple myeloma. The first patient treated with CAR-T therapy has been disease-free for 11 years.
In recent years, China has made significant advances in the treatment of hematologic malignancies. The establishment of the “Chinese Expert Consensus on the Diagnosis and Treatment of High-Risk Multiple Myeloma” and the presentation by Professor Huang He at the 2024 #EHA conference on targeting CD7 universal CAR-T therapy for T-cell acute lymphoblastic leukemia (T-ALL) and T-cell lymphoblastic lymphoma (T-LBL) have shown remarkable efficacy and safety. Additionally, exciting new data from the 2024 American Society of Clinical Oncology (#ASCO) annual meeting highlighted the efficacy of Relma-cel in treating relapsed/refractory large B-cell lymphoma (R/R LBCL), with a four-year overall survival rate (#OS) of 66.7%. Particularly noteworthy is the research on multiple myeloma, where the BCMA-targeted CAR-T therapy has demonstrated deep and lasting responses, with a complete response (#CR) rate of 82.4% and a 12-month progression-free survival (#PFS) rate of 85.5%.
With the continuous development of new treatments and the emergence of new drugs, hematologic malignancies in China are no longer considered incurable diseases. Through standardized, individualized, and precise treatments, many patients with hematologic malignancies can achieve long-term disease-free survival, and even a cure, returning to normal work and life. As medicine continues to advance, every life will continue to shine brightly!

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#HematologicMalignancies #LeukemiaAwareness #LymphomaResearch #MultipleMyeloma #BloodCancer #CancerResearch #CAR_Therapy #StemCellTransplant #Immunotherapy #TargetedTherapy #MonoclonalAntibodies #CancerTreatment #MedicalAdvancements #CancerSurvivor #HealthcareInnovation
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Expert Perspective: Side Effects and Management of CAR-T Therapy for a Thai Patient
**Expert Perspective: Side Effects and Management of CAR-T Therapy for a Thai Patient**
At the Hematology Department of Shanghai Tongji Hospital, Dr. Li Ping, the chief physician for the elderly Thai patient Ms. P, provided a detailed overview of the patient’s multiple myeloma condition and treatment journey. After experiencing multiple treatments and relapses in Thailand, the patient ultimately chose and trusted CAR-T therapy in China. Dr. Li highlighted that the most common side effect is cytokine release syndrome (CRS), which manifests as fever, hypotension, and difficulty breathing. While most CRS cases are mild to moderate, severe CRS can be life-threatening. She also emphasized that through scientific management, the team’s extensive experience, and the low immunogenicity of the fully human CAR-T product FUCASO, the side effects of CAR-T therapy can be effectively controlled, offering the patient hope for a cure.
We will continue to follow up on this patient’s treatment progress and provide updates.
#CARTherapy #MultipleMyeloma #FUCASO #Equecel #TongjiHospital #Shanghai #MedicalInnovation #CancerTreatment #Hematology #PatientJourney #Immunotherapy
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
High Risk Multiple Myeloma Solution and Chinese Expert Consensus
**High Risk Multiple Myeloma Solution and Chinese Expert Consensus**

Multiple Myeloma
High risk multiple myeloma (HRMM) refers to patients with multiple myeloma whose overall survival is less than 2 to 3 years under current standard treatments.
In 2024, the Chinese Society of Clinical Oncology’s Multiple Myeloma Expert Committee and the Chinese Anti-Cancer Association’s Hematologic Oncology Committee, organized by relevant experts, developed the “Chinese Expert Consensus on the Diagnosis and Treatment of High Risk Multiple Myeloma (2024 Edition),” which was officially published in the *Chinese Journal of Hematology* in May 2024. This consensus defines HRMM, outlines high-risk factors and risk stratification systems, and provides key treatment recommendations for HRMM, aiming to improve the quality of life and prognosis for HRMM patients in China.
**Definition of HRMM**
There is currently no precise definition of HRMM. Referencing the International Myeloma Working Group (IMWG)’s definition, the Chinese Expert Committee considers HRMM patients as those with an overall survival (OS) of less than 3 years after receiving autologous hematopoietic stem cell transplantation (auto-HSCT) or less than 2 years if they have not received auto-HSCT. Patients with OS of less than 2 years after receiving auto-HSCT are classified as ultra-high risk multiple myeloma (UHRMM) patients.
**Prognostic Factors of HRMM**
The biological characteristics of MM tumor cells and treatment response are key determinants in identifying HRMM.
**Static Prognostic Factors of MM**
-
**Genetic High-Risk Factors:**
In the context of genetic high-risk factors, cytogenetic abnormalities are core indicators in the MM risk stratification system, but there is still some debate over the definition of high-risk cytogenetic abnormalities (HRCAs). The “National Comprehensive Cancer Network (NCCN) Guidelines (2024.v1)” indicate that the presence of multiple HRCAs correlates with a poorer prognosis. Fluorescence in situ hybridization (FISH) is currently the main genetic testing technique for MM, and next-generation sequencing can be performed if conditions allow (TP53 mutations have a significant impact on prognosis, while the effects of KRAS, NRAS, DIS3, BRAF, and FAM46C are less clear). All these genetic tests require enrichment and selection of plasma cells.
-
**Non-Genetic High-Risk Factors:**
Confirmed non-genetic prognostic factors include International Staging System (ISS) stage III, extramedullary disease excluding bone lesions, circulating plasma cells, high plasma cell proliferation index, elevated lactate dehydrogenase (LDH), frailty, renal insufficiency, and thrombocytopenia.
**Dynamic Prognostic Factors of MM**
-
**Duration of Initial Treatment Response:**
The duration of response to initial treatment is a crucial dynamic prognostic factor for MM. Patients who received auto-HSCT followed by maintenance therapy and experienced relapse/progression within less than 2 years are classified as HRMM; for those who did not receive auto-HSCT, relapse within less than 18 months after starting treatment also indicates HRMM. Functional high risk refers to MM patients without known genetic high-risk factors at diagnosis who experience early progression within 18 months after the start of treatment.
-
**Depth of Initial Treatment Response:**
The depth of response to initial treatment is another important dynamic prognostic factor for MM. Patients who achieve negativity for minimal residual disease (MRD) in both bone marrow and imaging studies have the best survival outcomes. Achieving MRD negativity can partially overcome the adverse prognosis associated with high-risk cytogenetics. Continuous dynamic MRD monitoring has greater clinical value than a single MRD result, as sustained MRD negativity for more than 12 months can translate into long-term survival.
The Expert Committee considers newly diagnosed MM to be classified as HRMM if any of the following criteria are met:
-
R-ISS stage III, extramedullary disease excluding bone lesions, presence of circulating plasma cells (plasma cell leukemia is defined as ≥5% plasma cells in peripheral blood), presence of one or more HRCAs [t(4;14), t(14;16), t(14;20), del(17/17p), 1q21 gain/amplification, del(1p32), TP53 mutation], although 1q21 gain alone does not define HRMM;
-
MM patients who have received auto-HSCT followed by maintenance therapy and experience relapse within less than 2 years from the start of treatment;
-
Patients who have not received auto-HSCT and experience relapse within less than 18 months from the start of treatment;
-
Functional high risk;
-
Extramedullary relapse/secondary plasma cell leukemia;
-
New occurrence of 1q21 gain/amplification and/or del(17/17p)/TP53 mutation at relapse.
**Treatment of HRMM**
**Principles of Treatment for Newly Diagnosed HRMM**
The standard treatment for HRMM has not yet been established. The overall treatment strategy includes:
-
Utilizing combination therapies with drugs that have different mechanisms of action;
-
Aiming to eradicate all tumor clones, with the goal of achieving and maintaining MRD negativity both inside and outside the bone marrow;
-
Implementing a treatment strategy that adjusts based on the effectiveness of the therapy;
-
Acknowledging that current treatment outcomes for HRMM are still unsatisfactory, and encouraging the exploration of experimental therapies.
**Treatment for Newly Diagnosed HRMM Suitable for Transplantation**
-
**Induction Therapy Before Transplantation:**
For HRMM, induction therapy with the RVd regimen as a bridge to auto-HSCT has not met expectations in terms of depth of response and long-term prognosis, and achieving MRD negativity is more challenging compared to standard-risk patients. Some studies with novel drug-modified regimens have shown that patients with one HRCA receiving the KRd regimen (carfilzomib, lenalidomide, and dexamethasone) sequentially followed by auto-HSCT achieved similar MRD negativity rates and progression-free survival (PFS) as standard-risk patients, with no statistically significant difference. Meta-analyses indicate that incorporating a CD38 monoclonal antibody as part of the treatment backbone in early-line therapy provides clinical benefits for patients with HRCAs. For UHRMM patients, more intensive treatment regimens, such as the Dara-VRdC regimen (OPTIMUM/MUKnine study) and the Dara+KTD-PACE regimen (TT7 study), can be considered.
-
**Auto-HSCT:**
Tandem transplantation involves performing a planned second auto-HSCT within 3–6 months after the first. It is recommended that HRMM patients collect sufficient hematopoietic stem cells for two auto-HSCTs during the first mobilization. Regardless of the response achieved after the first transplant, it is advised to perform the tandem transplant within six months. The conditioning regimen for both transplants typically includes high-dose melphalan.
-
**Consolidation Therapy:**
If tandem transplantation is not performed, the original induction regimen can be continued for consolidation therapy for an additional 2–4 cycles.
-
**Maintenance Therapy:**
For HRMM patients, maintenance therapy should consider a combination of proteasome inhibitors, immunomodulators, and CD38 monoclonal antibodies, in either dual or triple drug regimens. It is recommended to continue maintenance therapy until disease progression or intolerance.
-
**Allo-HSCT:**
The long-term efficacy of allo-HSCT remains debatable and should only be considered within the context of clinical trials and for select high-risk patients.
#HRMM #AutoHSCT #CancerTreatment #MultipleMyeloma #InductionTherapy #TandemTransplantation #ConsolidationTherapy #MaintenanceTherapy #ProteasomeInhibitors #Immunomodulators #CD38MonoclonalAntibodies #AlloHSCT #UHRMM #NovelTherapies
Expert Consensus on the Treatment of HRMM
①Induction Therapy: For pre-transplantation induction therapy in HRMM, it is recommended to use a regimen based on CD38 monoclonal antibodies combined with proteasome inhibitors and immunomodulators. Recommended regimens include: Dara+KRd, Isa+KRd, Dara+VRd, and Isa+VRd. For patients who cannot tolerate a four-drug regimen, the KRd regimen is an alternative. For patients with significant extramedullary involvement (soft tissue or peripheral blood), additional cytotoxic drugs and, if necessary, radiotherapy can be added.
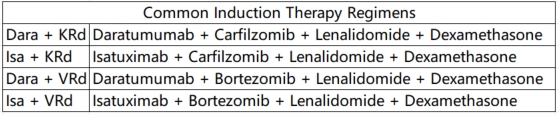
Therapy Regimens
②Auto-HSCT: Early auto-HSCT is the standard treatment for HRMM. For patients who receive auto-HSCT without significant adverse effects, tandem transplantation within six months post-transplant is recommended.
③Consolidation Therapy: For patients who do not undergo tandem transplantation, it is advised to continue consolidation therapy with the original induction regimen for 2–4 cycles.
④Maintenance Therapy: Maintenance therapy should involve a combination of proteasome inhibitors, immunomodulators, and CD38 monoclonal antibodies, either as dual or triple drug regimens. Therapy should continue until disease progression or intolerance.
⑤Clinical Research: Clinical studies targeting HRMM are encouraged, and it is recommended that HRMM patients prioritize enrollment in clinical trials.
**Treatment for Newly Diagnosed HRMM Not Suitable for Transplantation**
For MM patients not suitable for transplantation, an individualized treatment plan should be selected based on the patient’s fitness status score (IMWG GA score is recommended). For patients with good or moderate health, it is recommended to continue using the same regimen as for transplant-eligible patients. For frail patients, the VRd-lite (modified bortezomib + lenalidomide + dexamethasone) regimen and the DRd (daratumumab + lenalidomide + dexamethasone) regimen are currently the most commonly used first-line treatments.
**Treatment of Relapsed HRMM**
For patients with functional high risk and those defined as HRMM based on dynamic risk factors, re-induction regimens should include combinations of next-generation drugs or drugs with different mechanisms of action. Several clinical studies have shown that CAR-T cell (BCMA CAR-T) therapy can sustain efficacy in relapsed HRMM.
**Expert Consensus**
-
For relapsed HRMM patients, it is recommended to select combination regimens involving next-generation drugs or drugs with different mechanisms of action.
-
Patients with relapsed HRMM are encouraged to participate in clinical studies of CAR-T cell therapy or bispecific antibody immunotherapy.
**Summary**
For the treatment of HRMM, it is recommended to use multi-drug combination therapies with different mechanisms of action and bridge to auto-HSCT, aiming for deep and sustained MRD negativity and prolonging overall survival (OS) in patients. Currently, clinical trials of CAR-T cell therapy for newly diagnosed HRMM are being conducted, and combining auto-HSCT with CAR-T cell therapy can leverage the therapeutic benefits of both. Besides the BCMA target, CAR-T cell therapies targeting GPRC5D and FcRH5, as well as bispecific antibodies, have shown good efficacy in relapsed and refractory MM. New drugs and innovative diagnostic and therapeutic strategies, including immunotherapy, hold promise for overcoming the challenges in HRMM treatment.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#HighRiskMultipleMyeloma #HRMM #MultipleMyeloma #CancerTreatment #Hematology #Oncology #MedicalResearch #CancerCare #CancerAwareness #ChinaMedicalAdvances #AutoHSCT #MedicalConsensus #UHRMM #BloodCancer
#CancerPrognosis #GeneticRiskFactors #CancerResearch #MMTreatment #MRD #Hematology #CancerSurvival #PrognosticFactors
#CancerCriteria #Hematology #HighRiskMyeloma #PlasmaCellLeukemia #CancerRelapse #CytogeneticAbnormalities
#InductionTherapy #TandemTransplantation #ConsolidationTherapy #MaintenanceTherapy #ProteasomeInhibitors #Immunomodulators #CD38MonoclonalAntibodies #AlloHSCT #NovelTherapies
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese Medical Team: Sequential CD20 CAR-T Therapy Outperforms Salvage Chemotherapy for CD19 CAR-T Resistant B-Cell Lymphoma Patients
## Chinese Medical Team: Sequential CD20 CAR-T Therapy Outperforms Salvage Chemotherapy for CD19 CAR-T Resistant B-Cell Lymphoma Patients

CAR-T therapy
In recent years, with the application of CD19 CAR-T in refractory/relapsed B-cell lymphoma, we have encountered patients for whom CD19 CAR-T therapy was ineffective or who relapsed shortly after initial success. While research continues to explore factors that may affect the efficacy of CD19 CAR-T therapy, doctors and scholars are actively seeking more effective treatments for these patients. In June of this year, Professor Ke Xiaoyan and Professor Hu Kai from the Lymphoma and Myeloma Department of Beijing Gaobo Hospital published an article titled “Salvage CD20-SD-CART Therapy in Aggressive B-Cell Lymphoma After CD19 CART Treatment Failure” in the journal *Frontiers in Oncology*. This study provides new insights for treating B-cell lymphoma patients who have failed CD19 CAR-T therapy.
### Efficacy of Sequential CD20 CAR-T Therapy Exceeds Salvage Chemotherapy Post-CD19 CAR-T Failure
Regarding the innovation of this research, Professor Hu Kai pointed out that international scholars currently focus on chemotherapy (including new drugs), targeted drug therapy, and allogeneic hematopoietic stem cell transplantation for B-cell lymphoma patients who have failed CD19 CAR-T therapy. However, there is limited data on switching targets for a second CAR-T therapy. Our team summarized the treatment outcomes of 93 B-cell lymphoma patients who failed CD19 CAR-T therapy. Among them, 54 out of 93 (58%) chose sequential CD20-targeted second CAR-T therapy, and 39 out of 93 (42%) opted for chemotherapy combined with new drugs and targeted therapy. The results showed that the complete remission rate (CRR) in the sequential CD20 CAR-T group was significantly higher than in the chemotherapy group, with rates of 27.8% and 7.9%, respectively (P=0.03).
After a median follow-up period of 18.54 months, the sequential CD20 CAR-T group showed a significantly longer median progression-free survival (4.04 months vs. 2.27 months, P=0.0032) and median overall survival (8.15 months vs. 3.02 months, P<0.0001) compared to the non-CAR-T group. Multivariate analysis further confirmed that sequential CD20-targeted second CAR-T therapy is an independent factor associated with improved overall survival (HR 0.28, 95%CI: 0.16-0.51; P<0.0001) and progression-free survival (HR 0.46, 95%CI: 0.27-0.8; P=0.005).
### Safety of Sequential CD20-Targeted Second CAR-T Therapy
The study noted that the incidence of severe CRS (≥ grade 3) and ICANS (≥ grade 3) was relatively low during the sequential CD20-targeted second CAR-T therapy, at 9.2% and 7.4%, respectively, with complete recovery after treatment. Other adverse reactions were also controllable.
### Factors Affecting the Efficacy of Second CAR-T Therapy
We used a univariate regression model to evaluate factors affecting the response to sequential CD20-targeted second CAR-T therapy. An IPI score of ≥3 (OR 0.3, 95%CI: 0.10-0.93, P=0.04) was significantly associated with a lower overall response rate (ORR). Additionally, ECOG PS ≥3 (HR 3.12, 95%CI: 1.23-7.94, P=0.02) was associated with decreased progression-free survival and overall survival.
### Study Commentary
Professor Ke Xiaoyan highlighted that the Lymphoma and Myeloma Department at Beijing Gaobo Hospital has accumulated extensive experience with CAR-T therapy in recent years. As the number of CAR-T cases increases in real-world scenarios, CD19 CAR-T may cure half of the patients with relapsed/refractory B-cell lymphoma. For patients who fail CD19 CAR-T therapy, clinical problems become more complex. Our experience suggests that selecting CD20-targeted second CAR-T therapy based on the expression of surface antigens on recurrent tumors can still achieve better results than salvage chemotherapy, extend patient survival, and maintain controllable safety. Tumor burden and the patient’s physical condition remain factors affecting the efficacy of sequential CD20 CAR-T therapy.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#CAR-T #CancerTreatment #LymphomaResearch #MedicalAdvancements #CD19CAR-T #CD20CAR-T #Oncology #ClinicalResearch #CancerSurvivorship #MedicalBreakthroughs
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

What are the adverse reactions after CAR-T cell reinfusion?
 Interpretation of Hot Topics Conerning Mutiple Myeloma Patients:
Interpretation of Hot Topics Conerning Mutiple Myeloma Patients:
 “What are the adverse reactions after CAR-T cell reinfusion?”
“What are the adverse reactions after CAR-T cell reinfusion?”
 CAR-T细胞回输后有哪些不良反应?
CAR-T细胞回输后有哪些不良反应?

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#CART #Mutiplemyeloma #carttherapy #myeloma #leukemia #drug #RRMM #cancer #cancertreatment #tcell #cell #advancedmedical #AdvancedMedicineinChina
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Hope to Eradicate Lung Cancer, Colorectal Cancer, and Various Solid Tumors! China’s First Self-Replicating mRNA Vaccine JCXH-211 Successfully Launched~
Hope to Eradicate Lung Cancer, Colorectal Cancer, and Various Solid Tumors! China’s First Self-Replicating mRNA Vaccine JCXH-211 Successfully Launched~

mRNA Vaccine
Tumor immunotherapy, as an emerging method for diagnosing and treating tumors, has garnered widespread attention since its inception. Prior to the advent of tumor immunotherapy, the clinical treatment of solid tumors relied solely on traditional surgical removal, radiotherapy, and chemotherapy. Although these methods were effective, they came with significant drawbacks such as drug side effects and the risk of recurrence.
Since the 1990s, mRNA vaccines have been considered therapeutic vaccines. During the COVID-19 pandemic in 2020, mRNA vaccines were used in clinical treatments, saving countless lives. Today, as the advantages of mRNA vaccines are gradually being discovered, they are now applied in multiple fields.
The advent of China’s first self-replicating mRNA vaccine JCXH-211 undoubtedly marks a new breakthrough in the clinical diagnosis and treatment of various solid tumors in China.
**What is the mRNA Vaccine JCXH-211?**
JCXH-211 is the world’s first self-replicating RNA drug encoding human interleukin-12 (IL-12) to enter clinical trials. This srRNA vaccine delivers mRNA encoding IL-12 into the cytoplasm, continuously expressing IL-12 to enhance the body’s immune response against tumor cells.
In preclinical studies involving various mouse and PDX disease models, JCXH-211 has shown the ability to effectively kill tumor cells, eliminate distant tumors, and prevent tumor recurrence. Comprehensive GLP toxicology studies have also demonstrated good safety.
JCXH-211 has a broad range of indications and is expected to effectively treat multiple solid tumors. The anticipated prospects are as follows:
**1. Lung Cancer:**
As one of the leading causes of cancer death worldwide, lung cancer is often diagnosed at a late stage due to difficulties in early detection. JCXH-211, through continuous expression of IL-12 and activation of immune cells, is expected to effectively control the growth and spread of lung cancer cells.
**2. Colorectal Cancer:**
Current clinical treatments for colorectal cancer primarily involve surgery and radiotherapy/chemotherapy, which can remove visible tumors but have a high recurrence rate. Periodic administration of JCXH-211 may significantly reduce recurrence and improve patient survival.
**3. Soft Tissue/Chondrosarcoma:**
These tumors are highly invasive and cover a wide range of areas, often affecting surrounding soft tissues and progressing rapidly. Current treatments are limited to surgery and radiotherapy/chemotherapy, which, although somewhat effective, are not thorough, leading to high recurrence rates. JCXH-211 could become a new treatment method, enhancing the body’s immune response to combat these stubborn cancer cells.
**4. Skin Cancer:**
Clinical treatments for skin cancer mainly include surgery and radiotherapy. While most skin cancers have a good prognosis, the effects on intractable melanoma are poor. The expression of IL-12 can activate specific T cells, greatly aiding in the elimination of melanoma cells.
**High-Efficiency Activation and Safety of the mRNA Vaccine JCXH-211!**
mRNA vaccines not only encode antigens to induce specific immune responses against tumors but also possess inherent immunostimulatory properties. This dual mechanism allows JCXH-211 to activate the immune system more efficiently, enhancing the overall anti-tumor effect.
Moreover, the safety of mRNA vaccines is well-recognized. Since DNA is the genetic material of most species, including humans and viruses, and is transcribed into mRNA before being translated into proteins to perform functions in the body, injecting mRNA into the human body does not enter the cell nucleus, thereby eliminating the risk of genomic insertion mutations. Additionally, mRNA can be naturally degraded and excreted from the body, preventing accumulation. Thus, JCXH-211 presents lower potential risks and is safer.
Currently, clinical trials are progressing steadily. With the rapid advancement of tumor immunotherapy, JCXH-211 is expected to become a standard treatment for various solid tumors, offering hope to many cancer patients.

 To assess whether the condition is suitable for cancer vaccine or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for cancer vaccine or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#LungCancer #ColorectalCancer #SolidTumors #mRNAVaccine #JCXH211 #CancerTreatment #TumorImmunotherapy #MedicalBreakthrough #Biopharma #CancerResearch #HealthcareInnovation #CancerHope #MedicalScience #HealthTech #ClinicalTrials #CancerSurvivor #CancerAwareness #InnovativeMedicine #CancerVaccine #Vaccine #mRNA #JCXH
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Successful CAR-T Treatment for Advanced Gastric Cancer! Beneficial for Liver Cancer, Brain Tumors, Pancreatic Cancer, with a DCR of 96.1%
Successful CAR-T Treatment for Advanced Gastric Cancer! Beneficial for Liver Cancer, Brain Tumors, Pancreatic Cancer, with a DCR of 96.1%

Gastric Cancer
According to a report by People’s Daily on April 6, 2023, China has achieved another milestone in the CAR-T field. Recently, China Jiangxi Province successfully completed its first case of CAR-T cell therapy for solid tumors. This not only represents a significant breakthrough in new cancer treatment technologies in Jiangxi but also marks a major advancement in China’s CAR-T research and application.
China’s Jiangxi Achieves a Breakthrough in CAR-T Therapy for Solid Tumors
The patient, an advanced gastric cancer patient, had previously undergone chemotherapy combined with immune checkpoint inhibitor (ICI) treatment, but the disease was not well controlled. The tumor had widely metastasized to the abdominal cavity and multiple bones, accompanied by complications such as anorexia, abdominal distension, and severe recurrent reduction in blood cells, making the patient unable to tolerate chemotherapy.
With no other options, the patient received CAR-T cell reinfusion therapy on March 20, 2023, at the Oncology Center of The Second Affiliated Hospital of Nanchang University, with substantial support from the team at Peking University Cancer Hospital (Beijing Cancer Hospital). Fortunately, the patient has safely passed the peak period of cytokine release syndrome (CRS). Compared to before CAR-T treatment, tumor markers have decreased to normal levels, and the patient’s physical condition, mental state, and symptoms (such as abdominal distension and anorexia) have significantly improved. This marks a “zero breakthrough” for CAR-T cell therapy in solid tumors in Jiangxi Province of China, leaving a significant mark in the field of CAR-T research and application in China.
Peking University Cancer Hospital Showcases CT041 CAR-T at 2024 ASCO Conference with a Disease Control Rate of 96.1%
In addition to this fortunate case, the research team from Peking University Cancer Hospital showcased a domestically developed CAR-T product named “satri-cel” (development code CT041) at the recent 2024 American Society of Clinical Oncology (ASCO) conference. Satri-cel is a CAR-T cell therapy targeting Claudin18.2 (CLDN18.2) and is the only CLDN18.2 CAR-T cell therapy in the world approved for clinical trials (IND) in both China and the United States, targeting digestive tract tumors such as gastric cancer and pancreatic cancer.
The final results of the Phase 1 clinical trial (NCT03874897) were presented in an oral report at this conference. In patients with gastric cancer/gastroesophageal junction adenocarcinoma (GC/GEJ) treated with satri-cel monotherapy, the results showed a disease control rate (DCR) of 96.1% (49/51), an objective response rate (ORR) of 54.9% (28/51), a median overall survival (mOS) of 9.0 months, a median progression-free survival (mPFS) of 5.8 months, and a median duration of response (mDOR) of 6.4 months.
As early as May 2022, the team at Peking University Cancer Hospital had reported clinical cases of CT041 treating gastric cancer. A 57-year-old gastric cancer patient (Pt08), who had previously undergone three treatments, including PD-1 antibody, showed improvement after receiving CT041 CAR-T cell therapy. Visual inspection and CT scans indicated that the patient’s condition improved, with reduced umbilical tumor lesions, and the efficacy was still ongoing as of the date of the report.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#CART #CancerTreatment #GastricCancer #LiverCancer #BrainTumor #PancreaticCancer #MedicalBreakthrough #Oncology #Jiangxi #ChinaBiotech #CancerResearch #ASCO2024 #PekingUniversityCancerHospital #InnovativeTherapy #MedicalAdvancements #HealthNews
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

#EBMT / The Success Story of Equecabtagene Autoleucel: The World’s First Fully Human CAR-T for Multiple Myeloma
#EBMT / The Success Story of Equecabtagene Autoleucel: The World’s First Fully Human CAR-T for Multiple Myeloma

Multiple Myeloma
In recent years, CAR-T cell therapy has made groundbreaking progress in the field of relapsed/refractory multiple myeloma (RRMM), offering hope to patients struggling with limited treatment options. Recent data shows that Equecabtagene Autoleucel achieved an impressive complete response (CR) rate of 82.4% in Chinese clinical trials, garnering widespread attention for its outstanding efficacy.
### Breakthrough Research Findings
At the upcoming 50th Annual Meeting of the European Group for Blood and Marrow Transplantation (EBMT) in the UK, a study titled “Matching-Adjusted Indirect Comparison of Effective Characteristics Among Different BCMA Targeting CAR-T in Treatment of Relapsed or Refractory Multiple Myeloma (MAIC)” will be presented. This study compares the efficacy of four BCMA-targeted CAR-T products, revealing that Equecabtagene Autoleucel’s overall response rate (ORR) and CR rate are significantly superior to those of other comparators, especially in terms of CR rate.
### Clinical Performance of Equecabtagene Autoleucel
Equecabtagene Autoleucel is the world’s first fully human CAR-T product approved for marketing, having received priority review approval in China on June 30, 2023. The approval is based on results from the FUMANBA-1 Ib/II clinical study conducted across 14 centers in China. The study demonstrated that among 91 RRMM patients who had not previously received CAR-T therapy and had relapsed after multiple lines of treatment, Equecabtagene Autoleucel achieved a sCR/CR rate of 82.4%, with 97.8% of patients reaching minimal residual disease (MRD)-negative status.
### Pharmacodynamics and Pharmacokinetics Advantages
Compared to other BCMA-targeted CAR-T products, Equecabtagene Autoleucel shows significant advantages in pharmacodynamics and pharmacokinetics. Data indicates that Equecabtagene Autoleucel has a median time to response (TTR) of 15 days, and 62.3% of patients maintained CAR-T cell persistence for over 6 months. These findings suggest that Equecabtagene Autoleucel not only acts quickly but also remains effective in the body for an extended period, providing long-lasting therapeutic benefits.
### Structural Advantages Unveiled
Equecabtagene Autoleucel’s design includes unique structural advantages that minimize CAR-T cell exhaustion within the patient’s body. Research shows that Equecabtagene Autoleucel’s dissociation constant (Kd) is close to the natural dissociation kinetics of human T cells, with a dissociation time of about 6 minutes. This allows CAR-T cells to efficiently activate, kill, and proliferate within the body. In contrast, other BCMA-targeted CAR-T products have lower dissociation constants and longer dissociation times, which can lead to CAR-T cell exhaustion and reduced longevity.
### Long-Term Efficacy Outlook
Equecabtagene Autoleucel’s excellent performance in clinical trials brings new hope to RRMM patients. The long-term efficacy data in Chinese patients are particularly noteworthy. As time progresses, further clinical data will continue to validate Equecabtagene Autoleucel’s efficacy and safety, offering more effective treatment options for RRMM patients worldwide.
The success of Equecabtagene Autoleucel is not only due to its remarkable clinical efficacy but also the extensive scientific research and innovative technology behind it. This groundbreaking therapy sheds new light on the treatment of multiple myeloma and paves the way for future advancements in CAR-T cell therapy.
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#CART #MultipleMyeloma #EquecabtageneAutoleucel #CancerTreatment #Biopharmaceuticals #MedicalBreakthrough #Oncology #InnovativeTherapies #ClinicalResearch #HealthAdvancements
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

One Year After Approval: How Effective is China’s First BCMA-Targeted CAR-T Therapy in Treating Multiple Myeloma?
### One Year After Approval: How Effective is China’s First BCMA-Targeted CAR-T Therapy in Treating Multiple Myeloma?

Multiple Myeloma
“In the past 20 years, the treatment of multiple myeloma has advanced rapidly, thanks in large part to the swift progress in drug development. Over the past year, China’s first independently developed and the world’s first fully human BCMA-targeted chimeric antigen receptor T-cell (CAR-T) therapy was approved. In our real-world clinical applications, we have treated over 20 patients with an overall effectiveness rate of nearly 100%.”
On June 30, 2024, exactly one year after the approval of China’s first BCMA-targeted CAR-T therapy (Iquilonsen Injection), and also the world’s first fully human BCMA-targeted CAR-T therapy, how effective has the treatment been for patients? Professor Qiu Lugui, Director of the Lymphoma Treatment Center at the Chinese Academy of Medical Sciences Hematology Hospital, was interviewed by the People’s Daily Health Client.
“Multiple myeloma has a slow onset and early stages often show no obvious symptoms, making it easy to misdiagnose. To date, once diagnosed, the vast majority of patients experience one or more relapses, entering a refractory state, which is an incurable disease,” Professor Qiu Lugui told the People’s Daily Health Client.
Professor Qiu explained that current drugs for treating multiple myeloma fall into three categories: immunomodulators, proteasome inhibitors, and CD38 monoclonal antibodies. The indications for these drugs have gradually moved from refractory cases to frontline treatments, transforming multiple myeloma from a deadly disease with a median survival of around three years to a relatively controllable malignant hematological tumor with a median survival of 10 years or more after systematic multi-drug therapy.
CAR-T cell therapy is a cutting-edge technology for treating malignant hematological tumors. China’s independently developed fully human BCMA-targeted CAR-T drug (Iquilonsen Injection) is designed for multiple myeloma patients who have relapsed or whose disease remains uncontrolled despite traditional treatments including proteasome inhibitors and immunomodulators.
One particularly memorable case for Professor Qiu was a 70-year-old patient. “At that time, the patient was extremely weak and had already undergone all available treatments, including two types of immunomodulators, two types of proteasome inhibitors, CD38 monoclonal antibodies, and intensive chemotherapy, with no other effective options left,” recalled Professor Qiu. “However, the patient had a strong desire to live. Seeing his eager eyes, we couldn’t remain indifferent.”
“After confirming with the patient, we decided to proceed with the fully human BCMA-targeted CAR-T Iquilonsen therapy. One month after the treatment, the first evaluation showed complete remission. To date, the patient remains in complete remission,” said Professor Qiu.
“In real-world applications, we have treated over 20 patients with an overall effectiveness rate approaching 100%. However, due to the high cost of the drug and the fact that it is not covered by medical insurance, making the drug accessible remains a challenge,” Professor Qiu told the People’s Daily Health Client. “Currently, there are two methods to address this issue: one is to meet the needs of economically disadvantaged patients through commercial insurance; the other is to meet the needs of patients who meet the criteria for inclusion in CAR-T clinical research.”
“Additionally, in the year since the approval of China’s first CAR-T therapy for treating multiple myeloma, not only domestic patients but also patients from Europe, Asia-Pacific, Africa, and other regions have come to China for CAR-T treatment. Overall treatment driven by dynamic prognostic stratification will be the future path to cure for multiple myeloma patients both in China and globally,” said Professor Qiu.
#MultipleMyeloma #CARTTherapy #CancerTreatment #MedicalBreakthrough #BCMATargeted #ChinaHealthcare #InnovativeMedicine #PatientSuccess #GlobalHealth #Hematology #Immunotherapy
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
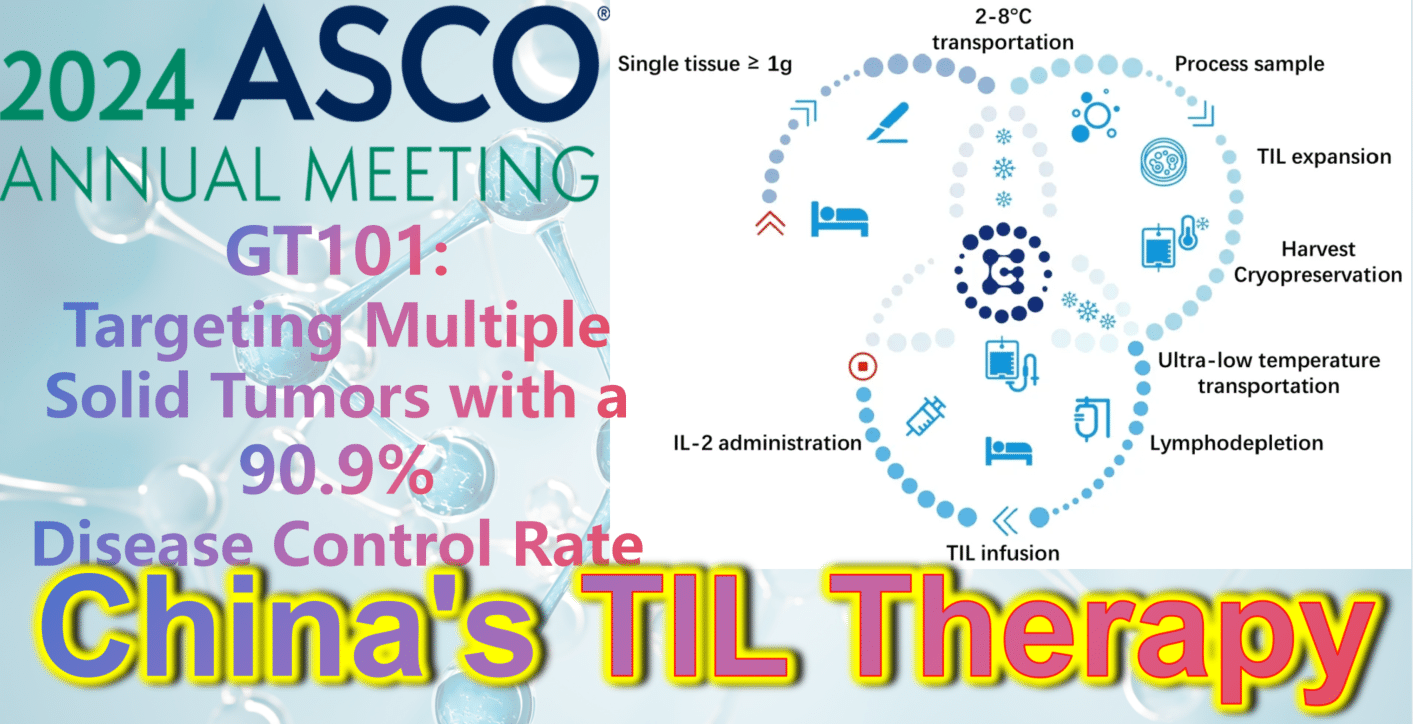
2024 ASCO China Highlights: China’s Indigenous TIL Therapy – GT101: Targeting Multiple Solid Tumors with a 90.9% Disease Control Rate
**2024 ASCO China Highlights: China’s Indigenous TIL Therapy Makes a Strong Debut, Targeting Small Cell Lung Cancer, Melanoma, Cervical Cancer, with a DCR Over 90%**
**GT101: Targeting Multiple Solid Tumors with a 90.9% Disease Control Rate**

TIL Therapy
