Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
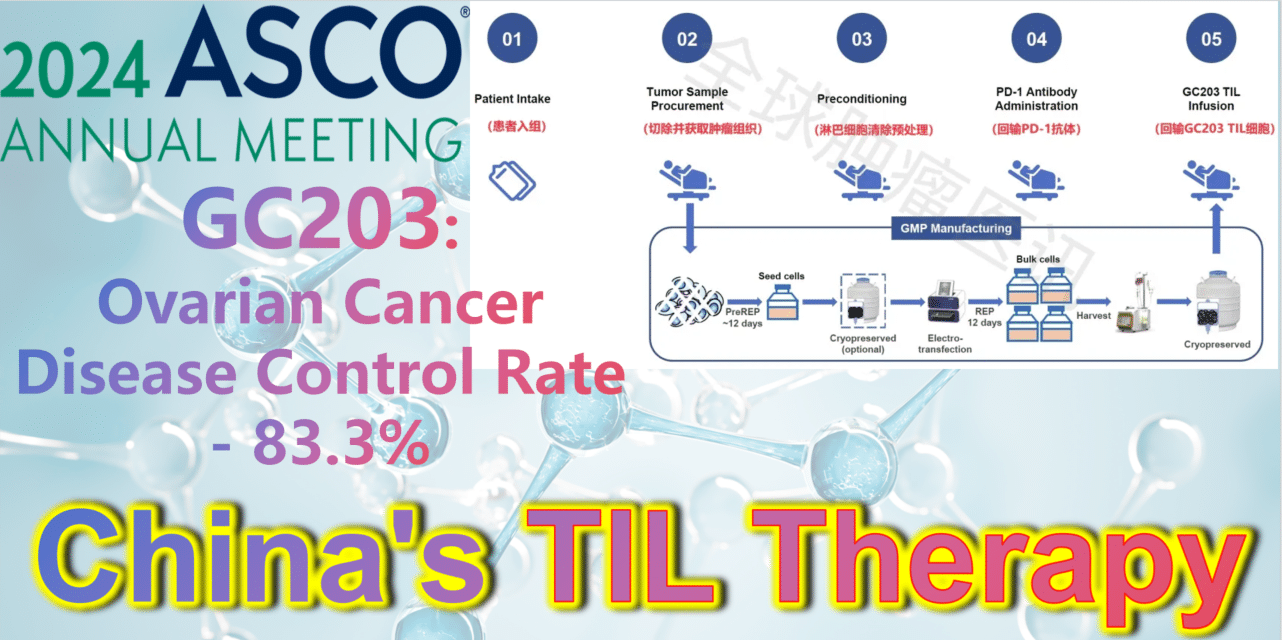
2024 ASCO China Voice: China’s TIL therapy – GC203: A Powerful Strike Against Ovarian Cancer with an 83.3% Disease Control Rate
2024 ASCO China Voice: China’s TIL therapy makes a grand debut, targeting ovarian cancer.
**GC203: A Powerful Strike Against Ovarian Cancer with an 83.3% Disease Control Rate**

TIL therapy
Ovarian cancer is a type of gynecologic tumor with a poor prognosis, with 70% of patients being diagnosed at a late stage. Unfortunately, effective treatment options for advanced ovarian cancer are quite limited, primarily relying on platinum-based chemotherapy. However, many ovarian cancer patients are not responsive to chemotherapy. Thus, there is an urgent need for new treatment options.
GC203 (mbIL-7-TIL) is a novel non-viral vector gene-modified TIL therapy utilizing membrane-bound IL-7. Developed by JunSai Biotech using the DeepTIL® cell expansion platform and NovaGMP® gene modification platform, it efficiently modifies T cells in a more economical way, enhancing the antitumor activity of TIL cells, activating internal immune cells, and avoiding systemic toxicity. It does not require lymphodepletion or combined IL-2 therapy post-infusion. A single patient is expected to save approximately 150,000 RMB in associated clinical costs, significantly improving the accessibility of TIL therapy and benefiting more cancer patients.
At the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, the latest clinical study results of GC203’s Phase 1 trial (NCT05468307) were announced. Between September 2021 and January 2024, 20 patients with recurrent ovarian cancer were enrolled, having undergone a median of 2.5 lines (range 1-9) of chemotherapy regimens (including PARP inhibitors, immune checkpoint inhibitors, etc.). After enrollment, patients first underwent tumor tissue resection, which was transported to GMP for a 22-26 day preparation period. The cryopreserved infusion products were then returned to the clinical center. Finally, patients received lymphocyte depletion pretreatment (including cyclophosphamide and hydroxychloroquine), a one-time PD-1 antibody infusion, and GC203 TIL cell reinfusion therapy.
After a median follow-up of 8.7 months (range, 2.9-18.8 months), results from 18 evaluable patients showed the following:
-
**Objective Response Rate (ORR):** The ORR in evaluable patients (n=18) was 33.3% (95% CI: 16.3%-56.3%). Among them, 22.2% (4 patients) achieved partial response (PR), and 11.1% (2 patients) achieved complete response (CR).
-
**Disease Control Rate (DCR):** The DCR in evaluable patients (n=18) was 83.3% (95% CI: 60.8%-94.2%).
-
**Median Progression-Free Survival (PFS):** The median PFS was 5.5 months (range, 1.0-14.1 months).
-
**Overall Survival (OS) Rate:** The 6-month OS rate was 75.6% (95% CI: 57.4%-99.6%); the 12-month OS rate was 68.8% (95% CI: 49.3%-95.9%).
-
**Adverse Reactions:** Most treatment-emergent adverse events (TEAEs) were grade 1 or 2, with common adverse reactions including elevated C-reactive protein levels (33%), fever (33%), and fatigue (11%), which could be alleviated or cured with symptomatic treatment. No other serious adverse reactions were observed.
In summary, for patients with recurrent or metastatic ovarian cancer with limited treatment options, GC203 TIL cell reinfusion therapy has shown good efficacy. Due to low-intensity pretreatment and no need for combined IL-2 therapy, its safety is significantly improved compared to traditional TIL therapy.
**How to Seek Help from TIL Therapy?**
The good news is that several TIL therapy clinical trials are currently recruiting in China, primarily targeting various solid tumors such as non-small cell lung cancer, melanoma, cholangiocarcinoma, esophageal squamous cell carcinoma, head and neck squamous cell carcinoma, breast cancer, ovarian cancer, cervical cancer, endometrial cancer, fallopian tube cancer, urothelial cancer, and renal cancer.
Patients seeking help from TIL therapy can submit their complete treatment history, recent pathology reports, imaging examination reports, and discharge summaries to Advanced Medicine in China.
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#OvarianCancer #TILTherapy #CancerTreatment #ASCO2024 #GC203 #Immunotherapy #MedicalResearch #Biotech #Oncology #ClinicalTrials #CancerInnovation #JunSaiBiotech #TIL #CancerBreakthrough #PatientCare #MedicalAdvancements
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

The Journey of an Indian Cancer Patient Seeking Treatment in China: Gratitude to Be Brought Back to India
**The Journey of an Indian Cancer Patient Seeking Treatment in China: Gratitude to Be Brought Back to India**

patient story
 “Before coming to China for treatment, I was filled with worries. My condition was not optimistic, and I was afraid I might never see my family again… But after arriving here, everyone I met was so warm and welcoming. The doctors are not only professional but also incredibly patient. I am now truly, truly happy, and I will bring this gratitude back to India.” — Ms. Savita
“Before coming to China for treatment, I was filled with worries. My condition was not optimistic, and I was afraid I might never see my family again… But after arriving here, everyone I met was so warm and welcoming. The doctors are not only professional but also incredibly patient. I am now truly, truly happy, and I will bring this gratitude back to India.” — Ms. Savita
 This is the true story of Ms. Savita from India, a multiple myeloma patient. Her journey to seek medical treatment was long and arduous, and she was often moved to tears when recounting it.
This is the true story of Ms. Savita from India, a multiple myeloma patient. Her journey to seek medical treatment was long and arduous, and she was often moved to tears when recounting it.
 In 2012, Savita was diagnosed with multiple myeloma in India. After undergoing various treatment methods, her condition relapsed, and doctors estimated she had only six months to live. Desperate and with nowhere else to turn, she decided to seek hope in China.
In 2012, Savita was diagnosed with multiple myeloma in India. After undergoing various treatment methods, her condition relapsed, and doctors estimated she had only six months to live. Desperate and with nowhere else to turn, she decided to seek hope in China.
 At the Jiangsu Provincial People’s Hospital North Branch, Savita successfully joined a clinical trial and underwent T-cell therapy. One month after treatment, evaluation results showed that her lesions had almost completely disappeared, indicating significant improvement.
At the Jiangsu Provincial People’s Hospital North Branch, Savita successfully joined a clinical trial and underwent T-cell therapy. One month after treatment, evaluation results showed that her lesions had almost completely disappeared, indicating significant improvement.
 When she was first admitted, Savita was bedridden, suffering from severe bone pain, and required daily injections of strong painkillers. Now, she can live like a healthy person and even went on a trip to Shanghai with her husband, Inder.
When she was first admitted, Savita was bedridden, suffering from severe bone pain, and required daily injections of strong painkillers. Now, she can live like a healthy person and even went on a trip to Shanghai with her husband, Inder.
 Even more joyful is the fact that her daughter will be getting married next January. Reborn with a new lease on life, she is filled with hope for the future, and her joy is palpable.
Even more joyful is the fact that her daughter will be getting married next January. Reborn with a new lease on life, she is filled with hope for the future, and her joy is palpable.
 Savita’s husband, Inder, shared their medical journey in China with a group of multiple myeloma patients in India, and many are hopeful to come to China for treatment.
Savita’s husband, Inder, shared their medical journey in China with a group of multiple myeloma patients in India, and many are hopeful to come to China for treatment.
 Ms. Savita’s story is a journey filled with hope and gratitude. She will bring this gratitude back to India, inspiring more people to see the light of hope.
Ms. Savita’s story is a journey filled with hope and gratitude. She will bring this gratitude back to India, inspiring more people to see the light of hope.

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: 137 1795 9070
Email: doctor.huang@globecancer.com
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

22 Days from Desperation to Rebirth! Chinese CAR-T Therapy Creates Survival Miracle for Thai Multiple Myeloma Patient
22 Days from Desperation to Rebirth! Chinese CAR-T Therapy Creates Survival Miracle for Thai Multiple Myeloma Patient

Patient story
Subtitle: Fighting for Love! A story of miraculous rebirth after all treatment options failed for a late-stage multiple myeloma patient in Thailand, who underwent CAR-T therapy in China.
Preface: When all treatment options had been exhausted, cancer progressed rapidly, and doctors regretfully said that Ms. M had no response to any medication. This situation could only lead to palliative care, meaning Ms. M had no chance to fight this disease anymore; her life was now on borrowed time. Fortunately, Ms. M’s family found new hope in China – CAR-T therapy. With the companionship of her family, Ms. M came to China and underwent the world’s first fully human BCMA CAR-T therapy. In just 22 days after treatment, all tumors miraculously disappeared, achieving complete remission (CR), and Ms. M was given a new lease on life!
In June 2021, the life of 58-year-old Ms. M experienced persistent back pain for over months. After detailed examinations at the hospital,resulting in a diagnosis of multiple myeloma. To quickly control the condition, Ms. M received treatments including PCD, DVD, bortezomib and lenalidomide, autologous hematopoietic stem cell transplantation.
In June 2023, the disease relapsed and Ms. M did not respond to any of these treatments. Local doctors informed Ms. M’s family that apart from palliative care, they were powerless, indicating that Ms. M’s life was now on borrowed time! Just as Ms. M was in dire straits, on June 30, 2023, the world’s first fully human BCMA CAR-T therapy – Equecabtagene Autoleucel, was shockingly launched in China, becoming a lifesaving straw for her. Ms. M and her family decided to seek treatment in China.
In September 2023, Professor Li Ping’s team at Shanghai Tongji Hospital developed a personalized Equecabtagene Autoleucel(BCMA CAR-T) therapy plan for Ms. M.
On September 8, 2023, Ms. M finally officially entered the CAR-T treatment process. After all examination items met the requirements, single-cell collection was performed first.
Bridge therapy began on September 11, 2023;
Local radiotherapy to relieve bone pain started on September 24, 2023;
Fludarabine plus cyclophosphamide chemotherapy was administered from October 9-12, 2023;
Finally, on October 14, a small bag of milky-white liquid was infused into Ms. M’s body. The doctor said that inside were billions of “special” T cells that could precisely kill multiple myeloma cells. Once inside the body, they would initiate a mode of frenzied sweeping, wiping out cancer cells completely.
What shocked everyone was that the examination results 22 days after CAR-T treatment showed that Ms. M had achieved hematologic CR, meaning that no cancer cells were detected in her blood. Her pain and anemia were also reversed. It was simply a miracle!
Every day now is precious to Ms. M. She can go shopping, chat with her family, enjoy delicious food with her family, and travel around the world with them, returning to the happy times before she fell ill. The only difference is that Ms. M and her family visit a fixed destination every month now – Shanghai, China. They have become accustomed to hearing the sacred announcement from the doctors here: no cancer in her body, continuing remission.
This is a microcosm of countless cancer patients overcoming the disease. The emergence of CAR-T cell therapy has brought new hope to cancer patients. I believe that in the future, more patients will be able to regain a new life like Ms. M.
Reference:
[1]Yuting Yan, et al. Blood Adv. 2019 Oct 8;3(19):2895-2904.
[2] 2023 IMS. P-290.
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Interpretation of Hot Topics Conerning Relapsed/Refractory Mutiple Myeloma Patients
 Interpretation of Hot Topics Conerning Relapsed/Refractory Mutiple Myeloma Patients:
Interpretation of Hot Topics Conerning Relapsed/Refractory Mutiple Myeloma Patients:
What other treatment options are available for patients with relapsed Multiple Myeloma?
How effective is the new drug treatment for the first relapse of Multiple Myeloma?
What treatment options are available for initial relapse of multiple myeloma?
What are the treatment goals for Relapsed/Refractory Multiple Myeloma (RRMM)?
What treatment strategy should be adopted for RRMM in order to achieve maximum relief?
For Relapsed/Refractory Multiple Myeloma, what are the Grades of Response?
Expert Introduction
👩🔬Zhuang Junling
Associate Chief Physician Department of Hematology
Peking Union Medical College Hospital
Published over 50 papers in journals such as Blood, Leukemia Research, and others.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#CART #Mutiplemyeloma #CARTtherapy #myeloma #leukemia #drug #RRMM #cancer #cancersurvivor #cancerpatient #bloodcancer #tumor #cartcell #cartcelltherapy #cancertreatment #advancedmedicineinchina #hematology
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Participants in China’s CAR-T ‘Lymphoma Value-Based Payment Plan’ have achieved complete remission
🎗️ **Participants in China’s CAR-T ‘Lymphoma Value-Based Payment Plan’ have achieved complete remission.** 🎗️

Lymphoma
Exciting news! Fosun Kite has just announced that the first participants in China’s “Lymphoma Value-Based Payment Plan” have achieved complete remission. These four patients, hailing from Shanghai, Guangzhou, and Hangzhou, have all reached complete remission after undergoing the innovative CAR-T cell immunotherapy, bringing new hope to lymphoma treatment.
### Why is CAR-T Therapy So Important?
Lymphoma is a malignant tumor originating from the lymphatic hematopoietic system, with diffuse large B-cell lymphoma being the most common type. For many patients, traditional treatment methods may be limited, especially for those with refractory or early-relapsed disease. The advent of CAR-T cell therapy offers these patients a new treatment option.
### Innovative Payment Model: Value-Based Payment
Fosun Kite’s “Value-Based Payment Plan” is a groundbreaking initiative. Eligible patients who do not achieve complete remission after treatment can receive up to 600,000 RMB in financial reimbursement. This payment model not only alleviates the financial burden on patients but also ensures the effectiveness and value of the treatment.
### Success Stories from Initial Participants
At the First Affiliated Hospital of Zhejiang University School of Medicine, one of the initial participants achieved complete remission after receiving CAR-T therapy. Deputy Director Hu Yongxian explains that when lymphoma cells can no longer be detected in the patient’s body, it signifies complete remission (CR), which is crucial for long-term survival and cure.
### Future Prospects
Professor Zhu Zhigang believes this innovative payment model can benefit more patients and potentially facilitate the inclusion of CAR-T therapy in national health insurance, further enhancing medical accessibility. In the future, we hope to see more patients gain new life through this advanced treatment.
### Conclusion
The emergence of CAR-T cell therapy and the introduction of the “Value-Based Payment Plan” bring unprecedented hope to lymphoma patients. This is not only a breakthrough in medical technology but also an innovation in medical payment models. Let’s look forward to more patients benefiting from this advanced treatment and embarking on the path to recovery.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#Health #CancerTreatment #InnovativeTherapy #CART #FosunKite #Lymphoma #MedicalInnovation #CompleteRemission
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Key Steps in Precision Treatment: A Detailed Overview of CAR-T Infusion for a Russian Patient at Jiahui in Shanghai
Key Steps in Precision Treatment: A Detailed Overview of CAR-T Infusion for a Russian Patient at Jiahui in Shanghai**
Vladimir Borzenkov, a 68-year-old patient from St. Petersburg, Russia, recently completed the core phase of CAR-T therapy—reinfusion—at Jiahui International Hospital in Shanghai. This crucial step involves reinfusing T-cells, which have been genetically modified to recognize and attack specific cancer cells, back into the patient’s body. For Mr. Vladimir, who has been battling his condition for over five years, this step is particularly significant.
Before the reinfusion, the medical team conducted a comprehensive physical assessment to ensure his body could withstand the procedure. Despite being in his sixties, Vladimir’s determination and desire for a cure helped him overcome any obstacles posed by his age. His journey began in December 2017 with back pain, which progressively worsened over several months. By February 2018, multiple bones, including his spine, sternum, and clavicle, showed signs of deterioration. Although this period seemed like a relentless trial, Vladimir never lost his faith in life. Now, at this critical juncture, he is filled with hope for the future.
For this treatment, Jiahui Hospital utilized FUCASO (Eque-cel), a fully human CAR-T therapy known for its unique low immunogenicity and lasting efficacy, providing therapeutic advantages for the patient. During the reinfusion process, Jiahui Hospital demonstrated advanced capabilities in monitoring and managing adverse reactions. The hospital is equipped with the latest monitoring technology and a highly experienced medical team, ensuring that any potential adverse reactions are promptly identified and managed, thus ensuring the safety and effectiveness of the treatment. This high level of monitoring and detailed attention to the patient’s health made Vladimir’s treatment process not only efficient but also safe.
Through his experience at Jiahui International Hospital, Vladimir witnessed the powerful potential of CAR-T therapy and the significant advantages of FUCASO. He is now filled with hope for the future treatment outcomes. This treatment marks not only a significant milestone in his personal battle against cancer but also showcases Jiahui’s expertise in CAR-T technology and excellent patient care.
#CART #MultipleMyeloma #FUCASOJourney #Equecel #JiahuiHospital #Shanghai #CancerSurvivor #bloodcancer #Immunotherapy #FullyHumanCART #CancerTreatment
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

International Standards for Innovative Medical Services: The Initial Experience of Singaporean Patients at Jiahui International Hospital
Title: International Standards for Innovative Medical Services: The Initial Experience of Singaporean Patients at Jiahui International Hospital
Content:
Singaporean patient, Ms. Teresa, recently experienced unique CAR-T therapy at Jiahui International Hospital in Shanghai. From scheduling to consultation, Jiahui Hospital demonstrated its top-notch service and medical expertise. During her initial visit, Teresa was impressed by the hospital’s modern facilities and the professionalism of the medical team.
The comprehensive assistance provided by the hospital, from language translation to medical consultation, ensured her comfort and peace of mind throughout the entire treatment process. Teresa shared, “From the moment I stepped into Jiahui, every meticulous care made me feel warm.” This visit not only strengthened her trust in Chinese medical services but also laid a solid foundation for her subsequent CAR-T therapy journey.
We will continue to monitor the progress of the patients’ treatment and provide follow-up reports.
#CART #CARTTherapy #HopeReborn #FUCASOApproval #Equecel #MultipleMyeloma #JiahuiHospital #Shanghai #Immunotherapy #MedicalInnovation #MedicalBreakthrough #CancerTreatment #FullyHumanCART
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

创新医疗服务的国际标准:新加坡患者在嘉会医院的初体验
创新医疗服务的国际标准:新加坡患者在嘉会医院的初体验
新加坡患者Teresa女士近期在上海嘉会国际医院体验了独特的CAR-T治疗。从预约到接诊,嘉会医院展示了其一流的服务和医疗水平。首次就诊中,Teresa对医院的现代化设施和医护团队的专业性印象深刻。医院提供的全面协助,从语言翻译到医疗咨询,确保了她在整个就诊过程中的舒适与安心。Teresa分享道:“从第一次步入嘉会,每一次细致的关怀都让我深感温馨。”此行不仅强化了她对中国医疗服务的信任,也为她后续的car-t治疗之路打下了坚实的基础。
我们将持续关注患者的治疗后续,并跟进报道。
#CART #CARTTherapy #HopeReborn #FUCASOApproval #Equecel #MultipleMyeloma #JiahuiHospital #Shanghai #Immunotherapy #MedicalInnovation #MedicalBreakthrough #CancerTreatment #FullyHumanCART
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese Innovation in Medicine: A New Milestone in Small Cell Lung Cancer Treatment!

 **Chinese Innovation in Medicine: A New Milestone in Small Cell Lung Cancer Treatment!**
**Chinese Innovation in Medicine: A New Milestone in Small Cell Lung Cancer Treatment!**


Small Cell Lung Cancer
#SmallCellLungCancerTreatment #BevosubabSingleMonoclonalAntibody #NewDrugApproval #MedicalBreakthrough #cancer #cancertreatment #lung #lungcancer
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Exploring CAR-T Therapy: Advantages and Challenges
Exploring CAR-T Therapy: Advantages and Challenges

CAR-T Therapy
CAR-T therapy, as an emerging immunotherapy approach, is gradually attracting attention. Its advantages in the field of cancer treatment bring hope, yet it also faces challenges that need to be overcome.
### Advantages:
**1. Powerful Antitumor Effect:**
CAR-T therapy modifies a patient’s T cells to enhance their ability to attack cancer cells. This customized treatment method can precisely identify and destroy tumor cells, offering new treatment opportunities for patients.
**2. Long-Term Survival:**
CAR-T therapy has a longer survival time, reducing the frequency of treatment for patients, thus reducing the inconvenience and pain associated with treatment, while also providing assurance of sustained efficacy.
**3. Low Side Effects:**
Compared to traditional chemotherapy and radiation therapy, CAR-T therapy has relatively low side effects. This reduces the occurrence of severe systemic toxicity reactions and central toxicity reactions, making patients more tolerable to the treatment process.
**4. Significant Treatment Effects in Blood Cancer:**
CAR-T therapy has achieved remarkable achievements in the field of blood cancer. Whether it’s acute lymphoblastic leukemia or multiple myeloma, it has shown significant efficacy, bringing new vitality to patients.
### Challenges:
**1. Risk of B-Cell Depletion:**
CAR-T therapy may lead to a decrease in the number of B cells in the patient’s body, increasing the risk of infection, requiring close monitoring and management.
**2. Off-Target Effects:**
Although CAR-T therapy is highly specific, there is still a risk of damaging normal cells, necessitating further improvement in treatment precision.
**3. Cytokine Release Syndrome (CRS):**
CAR-T therapy may trigger CRS, leading to severe immune reactions that require timely intervention.
**4. Neurotoxicity:**
Neurological symptoms may occur during treatment, requiring timely identification and management to reduce adverse effects on patients.
**5. High Treatment Costs:**
The production cost of CAR-T therapy is high, limiting its widespread clinical application, necessitating the search for cost-reduction methods to benefit more patients.
Although CAR-T therapy faces some challenges, with the continuous progress of science and technology and the deepening of research, we believe that these issues will gradually be resolved. As an innovative treatment method, CAR-T therapy brings new hope to cancer patients. We look forward to its greater role in the future, bringing better quality of life and treatment outcomes to patients. 🌟
#CAR-Ttherapy #CancerImmunotherapy #MedicalTechnologyInnovation
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Exploring China’s Indigenous Drug Anlotinib: A Beacon of Hope for Digestive Tract Tumor Patients
Exploring China’s Indigenous Drug Anlotinib: A Beacon of Hope for Digestive Tract Tumor Patients

China’s Indigenous Drug
In the continuous advancement of medicine, Anlotinib combined chemotherapy is emerging as a frontline therapy for advanced, unresectable liver metastases from digestive tract tumors. Let’s delve into the latest findings from a phase II clinical trial initiated by researchers from the Shanghai Jiao Tong University School of Medicine Affiliated Ruijin Hospital.
ALTER-G-001 Cohort C: A Glimmer of Hope
As of November 13, 2023, a total of 41 patients were enrolled in ALTER-G-001 Cohort C, comprising mainly 29 cases of pancreatic cancer, 6 cases of gastric cancer, and 5 cases of biliary tract cancer. These patients had a median age of 64 years, with 63.4% being male, and 92.7% having an ECOG performance status of 1. 56.1% of patients had liver metastases only. All patients received Anlotinib combined with standard chemotherapy. The efficacy assessment revealed partial responses in 16 patients and stable disease (with tumor shrinkage in 14 patients) in 17 patients, resulting in an objective response rate (ORR) of 42.1% and a disease control rate (DCR) of 86.8%. Among evaluable pancreatic cancer patients, the ORR and DCR were 42.3% and 88.5%, respectively. These data underscore the significant efficacy of Anlotinib in treating digestive tract tumors.
Safety and Tolerability
Regarding safety, 39 patients experienced treatment-related adverse events (TEAEs), with 53.7% of patients experiencing ≥Grade 3 TEAEs. Major adverse events included neutropenia (19.5%), leukopenia (14.6%), and thrombocytopenia (9.8%). However, despite some adverse events, the overall safety profile of Anlotinib combined chemotherapy remains acceptable.
Looking Ahead
Anlotinib, developed independently by the Chinese pharmaceutical company CTTQ PHARMA, is changing the treatment landscape for patients with advanced liver metastases from digestive tract tumors. Apart from pancreatic cancer, Anlotinib is undergoing clinical trials for various cancers, including non-small cell lung cancer, soft tissue sarcoma, gastric cancer, colorectal cancer, medullary thyroid carcinoma, differentiated thyroid carcinoma, and esophageal squamous cell carcinoma. This brings more hope and options for patients.
Conclusion
Anlotinib combined chemotherapy as a frontline treatment demonstrates good efficacy and acceptable safety for patients with advanced liver metastases from digestive tract tumors, bringing a ray of hope. We look forward to further research on this therapy and more patients benefiting from it in the future. May every patient find healing and hope in the progress of medicine.
We have access to medical resources from all cancer hospitals in China and can assist patients in receiving treatment from the best and top-tier oncologists in the CHINA.
doctor.huang@globecancer.com
WhatsApp:+8613717959070
#Anlotinib #DigestiveTractTumors #CancerTreatment #MedicalAdvancements
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Introducing the Momentum of the Next-Generation “Pan-Cancer” Targeted Therapy ICP-723 of China
 Introducing the Momentum of the Next-Generation “Pan-Cancer” Targeted Therapy ICP-723 of China
Introducing the Momentum of the Next-Generation “Pan-Cancer” Targeted Therapy ICP-723 of China

Pan-Cancer
ICP-723
 On April 11, 2022, InnoCare Pharma unveiled the preclinical data of ICP-723. The research results demonstrate that ICP-723 effectively inhibits the kinase activity of TRKA, TRKB, and TRKC (IC50 value less than 1 nM). Not only does ICP-723 exhibit potent in vitro efficacy in TRK-driven tumors, but it also overcomes resistance mutations commonly seen after treatment with first-generation TRK inhibitors, such as TRKA G595R and TRKC G623R/E.
On April 11, 2022, InnoCare Pharma unveiled the preclinical data of ICP-723. The research results demonstrate that ICP-723 effectively inhibits the kinase activity of TRKA, TRKB, and TRKC (IC50 value less than 1 nM). Not only does ICP-723 exhibit potent in vitro efficacy in TRK-driven tumors, but it also overcomes resistance mutations commonly seen after treatment with first-generation TRK inhibitors, such as TRKA G595R and TRKC G623R/E.
TGI 89.5%
 Further in vivo efficacy studies have confirmed the robust anti-tumor effect of ICP-723 in animal models. At a dosage of 1 mg/kg, ICP-723 achieves a tumor growth inhibition rate (TGI) of 89.5% in the KM12 tumor model. With high oral bioavailability, ICP-723 demonstrates overall favorable pharmacokinetic parameters.
Further in vivo efficacy studies have confirmed the robust anti-tumor effect of ICP-723 in animal models. At a dosage of 1 mg/kg, ICP-723 achieves a tumor growth inhibition rate (TGI) of 89.5% in the KM12 tumor model. With high oral bioavailability, ICP-723 demonstrates overall favorable pharmacokinetic parameters.
Next-Generation NTRK Inhibitor
 ICP-723, a Chinese developed next-generation NTRK inhibitor, holds promise in treating advanced or metastatic solid tumors harboring NTRK fusion genes, including breast cancer, colorectal cancer, lung cancer, thyroid cancer, and patients resistant to first-generation NTRK inhibitors like larotrectinib and entrectinib.
ICP-723, a Chinese developed next-generation NTRK inhibitor, holds promise in treating advanced or metastatic solid tumors harboring NTRK fusion genes, including breast cancer, colorectal cancer, lung cancer, thyroid cancer, and patients resistant to first-generation NTRK inhibitors like larotrectinib and entrectinib.
 On August 31, 2021, InnoCare Pharma announced the Investigational New Drug (IND) approval from the U.S. Food and Drug Administration (FDA) to initiate Phase I clinical trials of its second-generation pan-TRK inhibitor ICP-723 in the United States.
On August 31, 2021, InnoCare Pharma announced the Investigational New Drug (IND) approval from the U.S. Food and Drug Administration (FDA) to initiate Phase I clinical trials of its second-generation pan-TRK inhibitor ICP-723 in the United States.
In the major financial performance report released by InnoCare Pharma in 2022, the efficacy of ICP-723 was also disclosed.
