Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
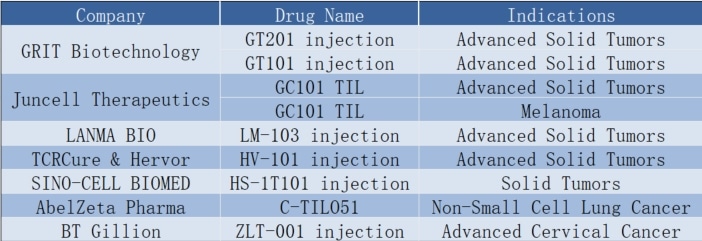
TIL Therapy: Revolutionizing Cancer Treatment Worldwide! China Accelerates into the Fast Lane!
🌟 **TIL Therapy: Revolutionizing Cancer Treatment Worldwide! 🌎** China Accelerates into the Fast Lane!
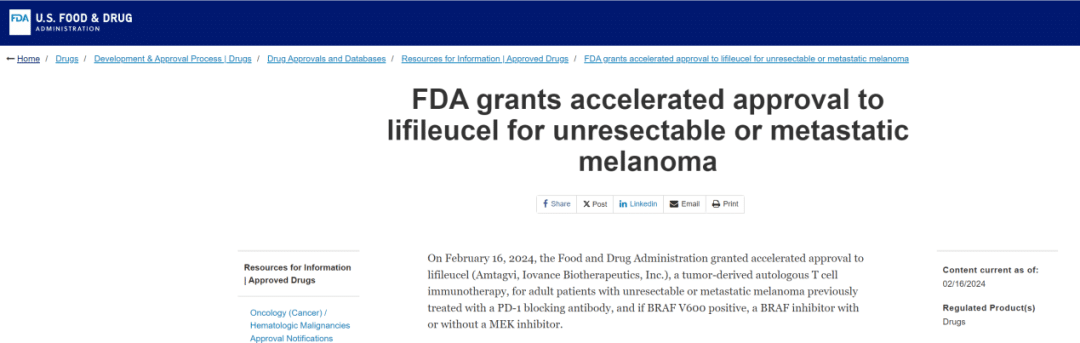
FDA

TIL therapy
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
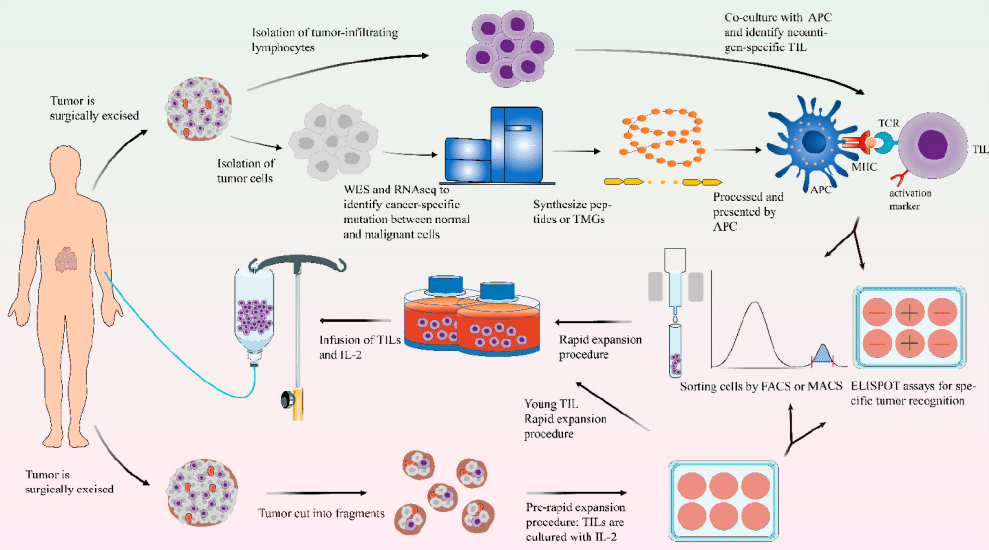
Unlocking the Potential: Understanding TIL Therapy for Solid Tumors
🔍 Unlocking the Potential: Understanding TIL Therapy for Solid Tumors🧬
💡What is TIL therapy?🧬
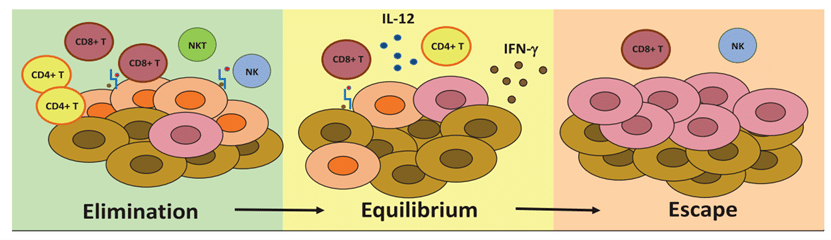
Cancer Development
Understanding Cancer Development: Immune Surveillance, Immune Equilibrium, and Immune Escape ���

TIL Therapy
🔬 The main routine steps of TIL therapy include:
💪Characteristics of TIL therapy:
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Breaking News: Another Milestone for Domestic CAR-T Therapy! Clinical Benefit Rate Reaches 71.4% in CT041 Trials, Challenging Gastric and Pancreatic Cancers with Astonishing Results!

Gastric Cancer, Pancreatic Cancer
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Breakthrough! Chinese Novel CAR-T Therapy Kills Tumors and Prevents Relapse!
🌟✨ **Breakthrough! Chinese Novel CAR-T Therapy Kills Tumors and Prevents Relapse!** ✨🌟

CAR-T Therapy
🔬 On January 2, 2024, a groundbreaking clinical study from China was published in *Nature*. This study, employing engineering design, enables CAR-T cells to secrete Interleukin-10 (IL-10), thereby enhancing metabolism within the tumor microenvironment. The modified IL-10 CAR-T cells increase oxidative phosphorylation in a mitochondrial acetoacetate carrier-dependent manner, resulting in complete regression of solid tumors and metastatic cancers, including colon cancer, breast cancer, melanoma, and pancreatic cancer. This breakthrough research offers new hope for cancer patients.
🌱 **The Miracle of IL-10** 🌱
The secretion of IL-10 promotes the proliferation and effector functions of CAR T cells, leading not only to the regression of solid tumors but also inducing stem cell-like memory responses in lymphoid organs, providing enduring protection against tumor re-attack. Specifically, IL-10 HER2 CAR-T cells achieved complete regression of MC38-HER2 tumors in mice, with a cure rate of 90%. In the case of melanoma, IL-10 TRP-1 CAR-T cells achieved a clearance rate of 60%, with significant success in treating the orthotopic B16F10 melanoma model.
🦠 **A Weapon against Relapse** 🦠
In addition to complete regression of solid tumors, IL-10 CAR-T cells demonstrated the ability to prevent relapse in immunodeficient mice. Mice treated with IL-10 CD19 hCAR-T cells for Raji or PANC1-CD19 tumors exhibited complete tumor regression without relapse, indicating stronger anti-tumor capabilities of IL-10 CAR-T cells in xenograft models. Particularly noteworthy is the effective elimination of pancreatic ductal adenocarcinoma (PDAC) tumors by IL-10 CD19 hCAR-T cells, resulting in complete response in all treated mice.
💊 **A Revolutionary Treatment Approach** 💊
These findings suggest that IL-10-expressing CAR-T cells are an effective immunotherapy against various solid tumors, capable of achieving complete regression in multiple synthetic and xenograft tumor models. What’s more exciting is that preliminary results indicate the metabolism-enhanced IL-10 CD19 CAR-T cell therapy developed by Leman Biotech requires extremely low treatment doses, consistently achieving complete remission in numerous relapsed/refractory lymphoma or leukemia patients, paving the way for a new era in cancer treatment.
✨ **A Beacon of Hope** ✨
This breakthrough study brings hope to cancer patients and demonstrates the immense potential of CAR-T therapy in cancer treatment. Looking ahead, further advancements in this technology promise to provide more opportunities for recovery and survival to patients worldwide. Let’s anticipate more breakthroughs together and strive towards conquering cancer
#all #CARTtherapy #CancerTreatment #RRMM #IL10 #Tumor #Nature #MedicalBreakthrough #CARTCELL #coloncancer #breastcancer #melanoma #pancreatic 🌟🔬💊
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Innovative Breakthrough: Chinese Team Creates Novel CAR-T Cells Using CRISPR/Cas9 Technology
🔬 Innovative Breakthrough: Chinese Team Creates Novel CAR-T Cells Using CRISPR/Cas9 Technology 🔬

Nature
💡 Analyzing the Scientific Principles behind the Technology 💡
🧬 Groundbreaking Clinical Validation 🧬
🌟 Pioneering a New Era in CAR-T Cell Therapy 🌟
💬 Expert Evaluation 💬
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Exciting News Alert! China’s New TCR-T Product IND Approved for Synovial Sarcoma!
🌟 Exciting News Alert! China’s New TCR-T Product IND Approved for Synovial Sarcoma! 🌟

synovial sarcoma

Cell Rep Med
China’s first TCR-T product IND approval takes aim at synovial sarcoma.
TAEST16001,

TCR-T Therapy
How to Seek TCR-T Therapy Assistance
or click on the WhatsApp+8613717959070
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
A New Era in Lung Cancer Treatment – Serplulimab Brings Innovative and Reliable Therapeutic Options!
**🌟 A New Era in Lung Cancer Treatment – Serplulimab Brings Innovative and Reliable Therapeutic Options! 🌟**

Cancer cell
📆CANCER CELL
🔬 Lung cancer
🌱NSCLC
🔍 ASTRUM-004
Serplulimab
🎉NEW ERA

👩🔬Lead
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese Hengrui Pharmaceuticals ‘s indication for cervical cancer has been approved for clinical trials.
🌟 Chinese Hengrui Pharmaceuticals ‘s indication for cervical cancer has been approved for clinical trials.🌟

cervical cancer

Atezolizumab

The Lancet Oncology
🌸🎀Atezolizumab injection has been studied in various fields, including small cell lung cancer, #NSCLC non-small cell lung cancer, esophageal cancer, liver cancer, and cervical cancer. It has shown promising efficacy in small cell lung cancer. Based on the results of the SHR-1316-III-301 study, the application for the market approval of Atezolizumab injection combined with chemotherapy as first-line treatment for extensive-stage small cell lung cancer has been accepted and approved in China in March 2023. The study results have been published in the top international medical journal “The Lancet Oncology,” and the original research from China has been internationally recognized.

Hengrui Pharmaceutical
🚑In addition to Cervical cancer, we are currently urgently recruiting patients with B-cell lymphoma, T-cell lymphoma, T-cell leukemia (T-ALL), acute lymphoblastic leukemia, myeloma, and other types of cancer!
You can send electronic copies or photos of genetic testing reports and diagnostic reports to the 📩email address: doctor.huang@globecancer.com📩, or click on the ✉️WhatsApp+8613717959070✉️ icon on the homepage. The Medical Department will contact you as soon as they receive the reports.
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese Exciting Good News Update in Lung Cancer Treatment! ORR: 78.6%
 Chinese Exciting Good News Update in Lung Cancer Treatment! ORR: 78.6%
Chinese Exciting Good News Update in Lung Cancer Treatment! ORR: 78.6%
 Meet Sunvozertinib, the game-changer in advanced lung cancer therapy!
Meet Sunvozertinib, the game-changer in advanced lung cancer therapy! 

Sunvozertinib, NSCLC

Lung Cancer
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Breakthrough in Cancer Treatment in CHINA: TX103 CAR-T Approved for Clinical Use Against Recurrent Brain Gliomas
🔬”Breakthrough in Cancer Treatment in CHINA: TX103 CAR-T Approved for Clinical Use Against Recurrent Brain Gliomas”🔬🧠

Brain Gliomas
✨ Exciting news in cancer research! Fuzhou Tuoxin Tiancheng Biotech’s TX103 CAR-T, a revolutionary therapy for recurrent stage 4 gliomas, has received clinical approval. 🌟 🧠
Article:

Tuoxin Tiancheng
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Revolutionizing Medicine: CAR-T Therapy Beyond Cancer
🚀Revolutionizing Medicine: CAR-T Therapy Beyond Cancer 🚀

🔬 Over the past decade, CAR-T cell therapy has transformed the field of oncology, successfully treating previously incurable blood cancers. While CAR-T therapy gained fame for its success in cancer treatment, the roots of this groundbreaking principle trace back nearly 30 years—initially exploring T cell therapy for HIV/AIDS. Although the early attempts didn’t succeed in treating HIV/AIDS, they demonstrated the enduring potential of engineered T cells in immunocompromised patients.
🚀 The future of CAR-T therapy holds vast potential in reshaping the landscape of medical treatment, reaching far beyond the realms of cancer. Stay tuned for ground
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China Leading Global Advances in Breast Cancer Immunotherapy
🌟China Leading Global Advances in Breast Cancer Immunotherapy 🌟#ChinaInMedicine #BreastCancer

Breast Cancer
Since 2020, breast cancer has surpassed lung cancer, becoming the most common cancer globally with approximately 2.3 million new cases and 680,000 deaths annually. Despite improvements in traditional treatments like surgery, chemotherapy, and radiation, the mortality rate remains high. China’s Chimeric Antigen Receptor (CAR) immunotherapy is making significant strides, spearheading innovation in breast cancer treatment. 🇨🇳💪
China is not only a trailblazer in breast cancer immunotherapy research but also a miracle creator, bringing new hope to cancer patients worldwide. Let’s witness China’s outstanding achievements in the medical field and acknowledge its contribution to global health! 💊🌏

