Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

🌟China Leading Global Advances in Breast Cancer Immunotherapy 🌟#ChinaInMedicine #BreastCancer

Breast Cancer
Since 2020, breast cancer has surpassed lung cancer, becoming the most common cancer globally with approximately 2.3 million new cases and 680,000 deaths annually. Despite improvements in traditional treatments like surgery, chemotherapy, and radiation, the mortality rate remains high. China’s Chimeric Antigen Receptor (CAR) immunotherapy is making significant strides, spearheading innovation in breast cancer treatment. 🇨🇳💪
Recent studies indicate substantial breakthroughs in CAR-T therapy, as well as CAR-NK cells and CAR macrophages for breast cancer treatment. The application of combination therapies further enhances the cytotoxicity of CAR-based cell therapies against breast cancer cells, bringing new hope to patients. 🚀
China is at the forefront of global clinical research on CAR, with 16 clinical trials focused on CAR-T or CAR-NK cell therapies currently underway. Particularly noteworthy is the research at the People’s Liberation Army General Hospital, where CAR-T cells targeting CD133 are employed to treat refractory advanced malignant tumors, including breast cancer. This approach has demonstrated excellent anti-tumor activity and manageable safety in patients previously treated for advanced hepatocellular carcinoma and cholangiocarcinoma. 🏥✨
On another front, the clinical study at Sun Yat-sen Memorial Hospital, Zhongshan University, is equally intriguing. They are exploring PD-1 knockout CAR-T cells targeting MUC1 for treating late-stage breast cancer patients. This trial is a dose-escalation exploratory study, and researchers anticipate its results to pave the way for future treatments. 🧪🔍
China is not only a trailblazer in breast cancer immunotherapy research but also a miracle creator, bringing new hope to cancer patients worldwide. Let’s witness China’s outstanding achievements in the medical field and acknowledge its contribution to global health! 💊🌏
#ImmunotherapyAdvancements #BreastCancerResearch
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
🌟2024 Lancet: Revolutionizing Cancer Treatment: China’s Breakthroughs in CAR T-Cell Therapy 🌏

LANCET
Dive into the cutting-edge world of CAR T-cell therapy, where China is making waves in the realm of cellular treatments. Since the inception of CAR T-cell clinical trials in 2013, this groundbreaking therapy has become a beacon of hope for cancer patients across the country.
🔬 Explosive Growth: By 2017, China led the globe in the number of CAR T-cell-related clinical trials, marking a pivotal moment in the evolution of cancer treatments. Fast forward to 2021, and Chinese cell therapy companies have amassed a staggering $237 million in funding, reflecting the robust expansion of CAR T-cell therapy in the nation.
🌐 Government Support: A deep dive into the research unveils the influential role of Chinese government policies in propelling CAR T-cell therapy forward. Strong governmental backing, coupled with capital influx, massive patient demand, and a unique healthcare system, lays the foundation for the accelerated growth of this revolutionary therapy in China.
🎗️ Overcoming Challenges: While CAR T-cell therapy is still in its infancy for solid tumors, it has achieved remarkable success in treating blood cancers. China has emerged as a global leader in conducting clinical trials, particularly focusing on hematologic malignancies like B-cell lymphomas. CAR-T cells, reprogrammed to combat cancer, offer a beacon of hope for those facing these formidable diseases.
🚀 Pushing Boundaries: Beyond blood cancers, Chinese researchers are actively exploring the potential of CAR-T cell therapy in various solid tumors. Preliminary studies hint at promising results for liver, pancreatic, and brain cancers. China’s commitment to medical innovation shines through as it pushes the boundaries of cancer research and treatment strategies.
🌈 Hope for the Future: Join us in celebrating the strides China is making in cancer research. Every breakthrough in CAR T-cell therapy brings us closer to a future where cancer is not just treated but conquered.
💪🏼🔬 #CARTCellChina #MedicalInnovation #CancerBreakthrough #HopeForTheFuture #GlobalHealthRevolution #CellTherapy #LANCET #HEMATOLOGY
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

The Chinese CAR-T therapy achieves a miraculous cure for advanced liver cancer, creating wonders in the field of cellular treatment for solid tumors.🌞🌞
🥰Surviving Against the Odds: A Chinese Doctor’s Journey with Liver Tumor🥰
Zou began his career in 1989 and has dedicated 30 years as an obstetrician-gynecologist, tirelessly working on the frontline of clinical care.
⭐️”One afternoon, I felt excruciating pain in my right shoulder while sitting in the office. It turned out I had three massive tumors on my liver. At that moment, I had no idea that my life had entered a countdown,” recalls Dr. Zou.⭐️
⭐️”Dr. Zou is in the advanced stage of liver cancer, with metastasis to the lungs,” urgently declared Prof.Shi as he convened a team of experts to consult on Dr. Zou’s condition.⭐️
⭐️ “With conventional treatment, the survival period is typically 3-6 months. Even with surgery, there’s no hope,” stated Prof.Shi.⭐️ Dr. Zou, a doctor himself, continued to ponder work matters, but colleagues and loved ones were tirelessly searching for any possible cure.
⭐️”Prof.Shi mentioned this immunotherapy, an antibody treatment,” said Dr. Zou’s wife.⭐️
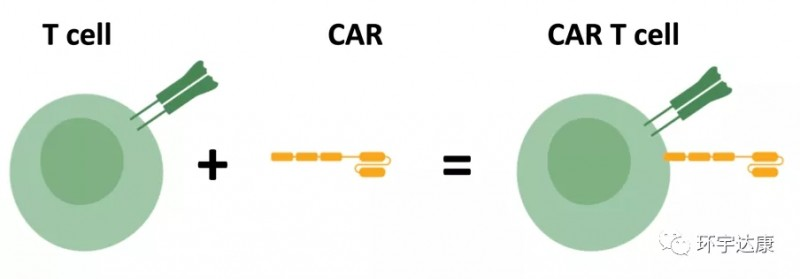
Antibody therapy, specifically Chimeric Antigen Receptor T-cell Immunotherapy (CAR-T), is a novel precision-targeted treatment for tumors. Using genetic engineering, T-cells are activated and equipped with a guided navigation device called CAR (Chimeric Antigen Receptor), transforming ordinary warriors into super soldiers known as CAR-T cells. These cells specifically recognize and efficiently eliminate tumor cells, achieving the goal of treating malignant tumors.
Fortunately, Dr. Zou’s health indicators perfectly matched the criteria for CAR-T therapy, providing him with a chance to defy death.
⭐️”In the early stages, I needed treatment every two weeks. Finally, after five or six sessions, my cancer cells started degenerating and liquefying. By now, the last and originally largest tumor has significantly liquefied and been absorbed,” Dr. Zou said.⭐️
⭐️”A miracle happened. We have taken a significant step in overcoming liver cancer in humans,” Prof.Shi added.⭐️
As the treatment gradually took effect, Dr. Zou’s health improved, allowing him to resume his daily work. After completing the treatment cycle, Dr. Zou once again donned the white coat, transitioning from patient to doctor.
In his heart, a stronger belief in the sincerity towards the medical profession and the health of patients emerged.
#SurvivorStories #MedicalMiracle #Inspiration #CancerSurvivor #CARTTherapy #AgainstAllOdds #HealthJourney #BelieveInMiracles #advancedmedicineinchina #chinesemedicine
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
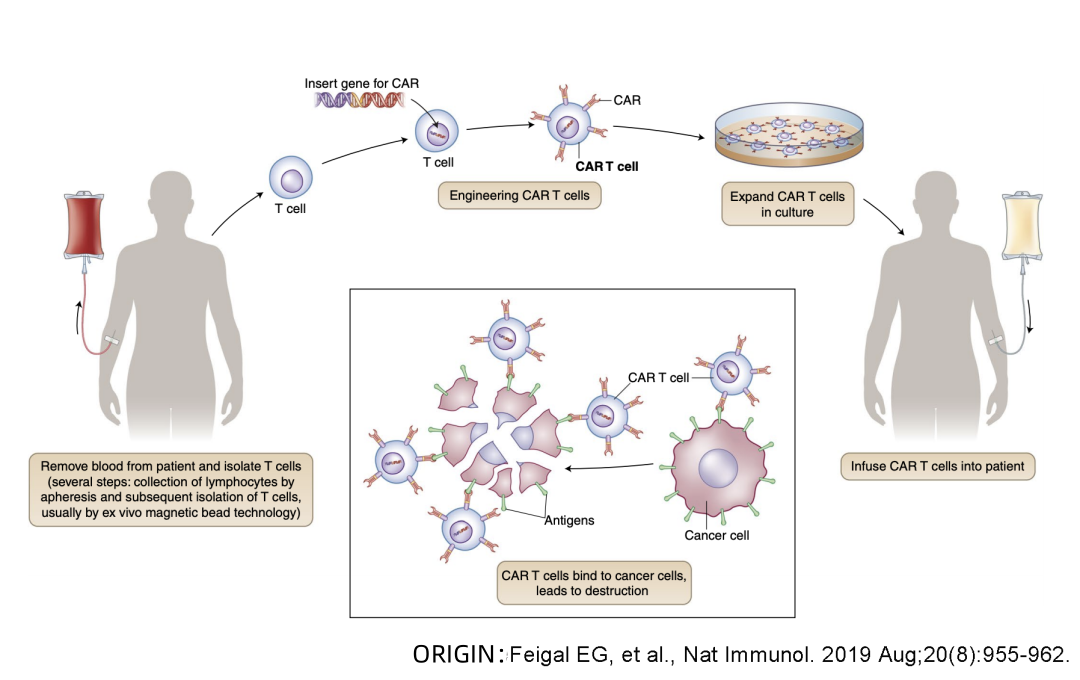
Beacon of Hope in Cancer Treatment
😊😊😊 What is the treatment process for CAR-T cell therapy?
CAR-T cell therapy has emerged as a global miracle in cancer treatment, capturing widespread attention. By loading CAR onto T cells, these “super soldiers” can specifically identify and efficiently eliminate tumor cells, offering a fresh perspective for cancer patients.
💙Assessment: Doctors first evaluate whether the patient is suitable for CAR-T therapy, ensuring maximum benefit.
❤️Isolation and Sampling: T cells are extracted from peripheral blood, preparing for subsequent modifications.
💛Cell Modification: T cells are activated and modified in vitro, using biotechnological techniques to load the CAR structure for specific tumor cell recognition.
🧡Ex Vivo Expansion: Depending on the patient’s weight and treatment period, CAR-T cells are significantly expanded ex vivo to ensure an adequate quantity for treatment.
💚Infusion into the Body: The expanded CAR-T cells are reintroduced into the patient’s body, allowing the “super soldiers” to combat tumors.
💜Subsequent Monitoring: Close monitoring of the treatment’s effectiveness post CAR-T cell infusion, promptly addressing any adverse reactions to ensure optimal patient outcomes.
The advent of CAR-T cell therapy brings a new dawn for cancer patients, with its unique treatment mechanism and remarkable effects positioning it as a leader in the field of cancer immunotherapy. This scientific marvel is spreading hope globally, opening a gateway to recovery for cancer patients.
#CARTCellTherapy #CancerTreatment #HopeInScience #CART #Cancer #Whatiscancer #cancerpatient
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

A Miraculous Journey: Israeli Artist Finds Cure for Multiple Myeloma in Hangzhou China
In a remarkable medical story, we turn our attention to the experiences of a friend seeking treatment in Hangzhou China. Tali, a well-known artist from Israel. In 2012, she was diagnosed with multiple myeloma, a malignant blood disease, and after seeking treatment in various European countries, including Israel and France, she found no definitive cure. In a moment of despair, she discovered a solution from Hangzhou, China, through the European Bone Marrow Transplant Association.

On October 6th, Tali and her family boarded a flight from Israel to Hangzhou. Under the meticulous care of Professor Huang He and his team at Zhejiang University’s First Hospital, Tali underwent over a month of intensive treatment. Today, Tali is finally on the road to recovery, expressing her gratitude to the medical staff through her preferred medium – art.

In the hospital room, Tali and Professor Huang He share laughs and conversations. The weather looks promising, initially being skeptical about her health when arriving in Hangzhou, encouragement from the medical team reignited her hope for recovery. Using her artistic language, she documented every moment of the treatment process.
After undergoing treatment, the excruciating bone pain gradually lessened, indicating a positive turn in her health.

“I can feel my body improving bit by bit. Green cells are gradually replacing the red ones, and my bone pain has completely disappeared. There are no tumor cells in the bone marrow anymore – they have vanished entirely.” Tali expressed.

The advanced treatment utilized at Zhejiang University’s First Hospital involves cutting-edge blood cell separation technology. Lymphocytes are extracted and genetically engineered to attack malignant tumor cells, successfully curing multiple myeloma. This revolutionary technique is known as CAR-T cell therapy. Zhejiang University’s First Hospital stands out as one of the earliest and most experienced clinical research centers using this technology, making it a pioneer in the field.

In here we bring you this inspiring story from Hangzhou, where art, science, and the human spirit come together in a tale of triumph over adversity.

Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Breakthrough Ultra CAR-T Therapy Shows High Disease Control Rate of 85.7% in Advanced Platinum-Resistant Ovarian Cancer
As a skilled overseas social media writer, here’s a revamped article for international media promotion:
In a groundbreaking development, PRGN-3005, an Ultra CAR-T cell therapy, is making waves with an impressive disease control rate (DCR) of 85.7% in the treatment of advanced platinum-resistant ovarian cancer. Utilizing non-viral gene delivery technology, this therapy eliminates the need for ex vivo expansion, significantly reducing production time and costs. The ability to administer the treatment rapidly, as soon as the second day after non-viral gene transfer, not only lowers production time and costs but also enhances tumor targeting precision. This holds the potential to disrupt the current landscape of CAR-T cell therapy.
Published in the Journal of Clinical Oncology, the “Phase 1/1b Clinical Trial of PRGN-3005 in Recurrent or Refractory (r/r) Ovarian Cancer” reported promising outcomes. The study included 25 assessable patients with a median age of 64 (range: 38-76) who had undergone extensive pretreatment with a median of 8 prior therapeutic regimens. The patients were divided into three groups: intraperitoneal (IP) infusion (C1, n = 12), intravenous (IV) infusion (C2, n = 6), and intravenous cyclophosphamide low dose (IV LD, n = 7).
Post-treatment, 20% of all participants exhibited regression in at least one target lesion (evaluated by RECIST 1.1 criteria). Notably, the DCR for the IV LD group was an impressive 85.7%, with a 57% reduction in target tumor burden and an average 27.4% decrease in CA125. A patient in the IV LD group experienced a 28% reduction in target tumor burden after a second PRGN-3005 infusion at the 12-month mark. Importantly, no PRGN-3005-related dose-limiting toxicities, neurotoxicities, or ≥3-grade cytokine release syndrome (CRS) adverse reactions were observed.
These results underscore the favorable tolerability of PRGN-3005 Ultra CAR-T therapy in treating ovarian cancer, demonstrating an encouraging disease control rate (DCR) and an overall reduction in tumor burden.
While CAR-T therapy has shown great promise in treating hematologic malignancies and several products have been approved, its application in solid tumor treatment is still in the clinical experimental stage. Nevertheless, with the continuous evolution of CAR-T generations, the emergence of more prominent targets, and improvements in proliferation and cytokine release aspects, the conquest of advanced solid tumors by CAR-T therapy seems imminent. Numerous clinical trials are already underway, offering hope for complete remission in fortunate patients.
#PRGN3005 #CARTTherapy #OvarianCancer #MedicalBreakthrough #CancerTreatment #Ovarian
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
Breakthrough CAR-T Cell Therapy for R/R B-Cell ALL: A Game-Changer in Chinese Medical Innovation
ALL- Acute Lymphoblastic Leukemia
The Fourth China Hematology Development Conference – CAR-T Frontier Forum and the First CAR-T Cell Immunotherapy Summit (JCIS), held on January 5, 2024, in Tianjin.
Potential Best-in-Class: Inaticabtagene Autoleucel Redefining Long-term Outcomes for R/R B-ALL
Professor Ma Jun from the Harbin Institute of Hematology and Oncology shared notable progress in immunotherapy and cell treatment for Chinese ALL. Previously, the overall complete response (CR) rate for adult R/R ALL treatment in China was approximately 40%, with a mere 11% 3-year survival rate. The introduction of CAR-T cell therapy has been a paradigm shift, altering the long-term outcomes for R/R B-ALL patients.
Inaticabtagene Autoleucel demonstrates superior efficacy:
Higher overall response rates (ORR) at 3 months and beyond, with median duration of response (DOR) and overall survival (OS) surpassing other products.
Patients treated with Inaticabtagene Autoleucel exhibit similar long-term benefits in OS, whether or not they undergo subsequent transplantation.
Inaticabtagene Autoleucel boasts enhanced safety:
Lower incidence rates of grade 3 cytokine release syndrome (CRS), grade 3 immune effector cell-associated neurotoxicity syndrome (ICANS), and infusion-related mortality compared to other CAR-T products.
Moreover, in minimal residual disease-positive (MRD+) B-ALL patients, CAR-T cell therapy has shown significant progress. It eradicates MRD, improves survival rates, and may serve as a first-line consolidation therapy for CR patients, aiding in:
Reduced transplant requirements, mitigating transplant-related complications
Maintenance of long-term remission for those unsuitable for allo-HSCT or unwilling to undergo it
Improved overall survival
Lower intensity and duration of intensive chemotherapy, leading to shorter treatment times and enhanced compliance.
The Future Outlook: Believing in the potential of CAR-T cell therapy, it is anticipated that this innovative treatment will extend hope to currently incurable diseases such as solid tumors and brain tumors. The strides made in Chinese medical innovation, exemplified by Inaticabtagene Autoleucel, signal a promising future for the global landscape of CAR-T cell therapy.
#CARTRevolution #InaticabtageneAutoleucel #HematologicCancerTherapy #ClinicalBreakthrough #ChinaHematologyConference #JCIS #BAllTreatment #MedicalAdvancements #CancerResearch #TreatmentInnovation #InaticabtageneAutoleucel #Autoleucel #ChineseCART #CARTTherapy #cancer #Bloodcancer #MedicineinCHINA #Medicaltourismo #Advancedmedicine #cancertherapy #leukemia
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

“Clinical Breakthrough: Chinese CAR-T – Inaticabtagene Autoleucel Revolutionizing Hematologic Cancer Therapy”
On January 5, 2024, the Fourth Chinese Hematology Development Conference was convened in Tianjin, China, concurrently hosting the CAR-T Frontier Forum and the First CAR-T Cell Immunotherapy Summit (JCIS). The focus was directed towards the latest advancements and clinical applications of Inaticabtagene Autoleucel, presenting new pathways for standardized treatments.
Under the moderation of Professors Wu Depei and Hu Yu, Professor Wang Ying from the Institute of Hematology at the Chinese Academy of Medical Sciences presented a specialized lecture titled “Interpreting Key Clinical Data of Inaticabtagene Autoleucel.” Professor Wang highlighted the challenges faced by adult B-ALL patients in China, commonly treated with salvage chemotherapy ± hematopoietic stem cell transplantation. However, the median survival period is only 2-6 months, and targeted therapies yield a median survival of merely 7.7 months, necessitating an urgent need for more effective treatment methods. Recently, the Inaticabtagene Autoleucel infusion has been approved for treating adult R/R B-ALL patients.
Critical clinical research (NCT04684147) has revealed the substantial outcomes achieved with a single treatment of Inaticabtagene Autoleucel:
Rapid and profound remission: Within 3 months of treatment, the overall response rate (ORR) reached an impressive 82.1%, with a 100% negativity rate for minimal residual disease (MRD), showcasing the remarkable effects of Inaticabtagene Autoleucel within a short span.
Enduring remission: The 3-month post-treatment overall remission rate stood at 64.1%, with a 12-month sustained remission rate (DOR) of 80%. With a median follow-up of 8.0 months, the median relapse-free survival (RFS) period has not been reached. The one-year survival rates for overall infused patients, those achieving complete response (CR)/complete response with incomplete hematological recovery (CRi) within 3 months, and those reaching CR/CRi at 3 months were 67.9%, 72.0%, and 85.6%, respectively.
Good safety profile: The incidence rate of ≥3-grade cytokine release syndrome (CRS) was only 10.3%, and that of ≥3-grade immune effector cell-associated neurotoxicity syndrome (ICANS) was merely 7.7%. Moreover, patients recovered post-treatment without complications.
Furthermore, data from over 100 patients validated the clinical efficacy and safety of Inaticabtagene Autoleucel, affirming its robustness and offering renewed hope to a larger patient population. This breakthrough treatment provides a new avenue for tackling challenging diseases and is poised to revolutionize the landscape of hematologic cancer therapy.
#CARTRevolution #InaticabtageneAutoleucel #HematologicCancerTherapy #ClinicalBreakthrough #ChinaHematologyConference #JCIS #BAllTreatment #MedicalAdvancements #CancerResearch #TreatmentInnovation #InaticabtageneAutoleucel #Autoleucel #ChineseCART #CARTTherapy #cancer #Bloodcancer #MedicineinCHINA #Medicaltourismo #Advancedmedicine #cancertherapy
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Exploring Tumor Vitality: Chinese CAR-T Therapy Grants Patients Complete Remission
“Is it true? It’s so unbelievable!” said Chen, the “fortunate one.”
On June 22, 2021, the approval of China’s first CAR-T cell therapy product, Yescarta, marked a significant milestone in medicine. Behind this milestone lies the story of a patient named Chen, diagnosed with diffuse large B-cell lymphoma, which not only brought confidence to patients but also injected fresh belief into medical professionals.
Chen was diagnosed with diffuse large B-cell lymphoma in July 2019, underwent primary treatment, only to unfortunately relapse. Due to the insufficient efficacy of secondary treatment and considering the TP53 mutation, conventional treatment plans were inadequate. However, the approval of Yescarta on June 22, 2021, brought a glimmer of hope for patients. Under the meticulous planning of the expert team in the Hematology Department of Ruijin Hospital, Chen successfully underwent CAR-T cell infusion therapy on August 2.
One year after the evaluation of the treatment’s effectiveness, Chen’s condition still maintains complete remission. This achievement has brought immense hope and joy to patients with diffuse large B-cell lymphoma, strengthening the belief in the potential of CAR-T therapy among medical practitioners.
“This is not only good news for patients but also an encouragement and boost to us, clinical doctors in the field of hematology,” stated Professor Xu Pengpeng.
This case represents not only a medical breakthrough but also serves as an inspiration for individuals battling diffuse large B-cell lymphoma. The success of CAR-T therapy reveals new possibilities, offering a new pathway to break the limitations of the “mere six-month survival period” in cancer treatment. May more patients benefit from this breakthrough, offering hope for healing and long-term remission.
#CARTtherapy #TumorVitality #CancerTreatment #NewMedicalTechnologies #MedicalAdvancement #Bloodcancer #cancersuvivor #lymphoma