Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Clinical Breakthrough: Chinese CAR-T – Inaticabtagene Autoleucel Revolutionizing Hematologic Cancer Therapy


Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Exploring Tumor Vitality: Chinese CAR-T Therapy Grants Patients Complete Remission


Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
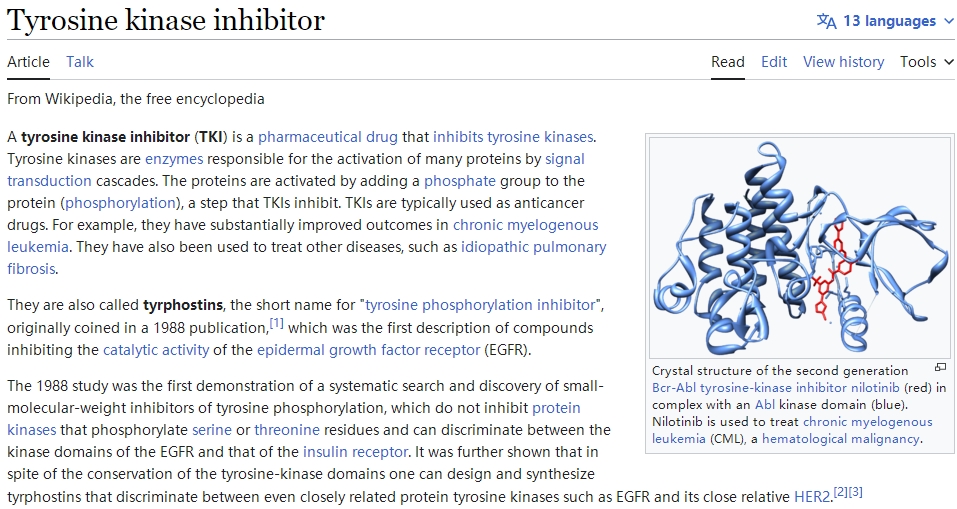
Breakthrough Advances in CAR-T Cell Therapy Combined with TKI for Malignant Hematologic System Tumors



Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Breakthrough in CAR-T Therapy Achieved by Chinese Sun Yat-sen University Cancer Center
Recently, Professor Huang Huiqiang’s team at Sun Yat-sen University Affiliated Cancer Hospital achieved significant progress in a lymphoma patient who relapsed after five lines of chemotherapy. Utilizing CAR-T therapy, the patient has experienced complete remission for over two years, making them one of the patients in China with the longest survival post-CAR-T treatment.

Lymphoma, one of the common blood cancers, has traditionally been treated using a combination of chemotherapy, autologous hematopoietic stem cell transplantation, targeted therapies, and immune-modulating drugs. However, CAR-T cell therapy, an advanced technology in the field of oncology, has emerged as a precise, rapid, and highly effective new treatment method in recent years.
Diffuse large B-cell lymphoma is a prevalent aggressive lymphoma characterized by rapid progression, high mortality rates, and short survival periods. Despite significant therapeutic advancements in recent years, approximately 70% of patients achieve good outcomes and long-term remission through first-line treatments, but around 30% of patients have inadequate responses to treatment, facing difficult-to-cure relapses.

Professor Huang Huiqiang emphasized, “CAR-T cell therapy plays a crucial role in providing new treatment hopes for relapsed and refractory patients who cannot undergo transplantation or have previously undergone ineffective treatments.
It is reported that in the future, Sun Yat-sen University Cancer Center will continue to explore clinical innovative technologies in CAR-T cell therapy, aiming to bring new prospects for numerous patients.
#CARTBreakthrough #CancerResearch #SunYatSenCancerCenter #InnovativeTherapies #MedicalBreakthrough #LymphomaTreatment #PrecisionMedicine #HopeForPatients #CancerSurvivorship #AdvancedOncology #MedicalInnovation #ResearchProgress #ScienceNews #HealthcareAdvancements #ImmunotherapySuccess #Cancer
Unveiling Hope: Chinese CAR-T Cell Therapy Illuminating New Paths in Liver Cancer Treatment!
2020, over 19.3 million people were diagnosed with cancer worldwide, leading to almost 10 million fatalities. In China alone, the number of new cancer patients reached a staggering 4.57 million, accounting for 23.7% globally. Liver cancer, among the most prevalent malignant tumors in China, witnessed 410,000 new cases and 380,000 deaths, making up 45.3% and 47.1% of the global total, respectively [1]. However, since the 21st century began, significant strides have been made in liver cancer treatments, particularly in medication and localized therapies. Surgical procedures are no longer the sole option for long-term survival among liver cancer patients.
Immunotherapy has emerged as one of the most promising techniques for treating liver cancer, especially with advancements in tumor molecular biology. In 2013, “Science” magazine categorized immunotherapy as the fourth major cancer treatment, following surgery, chemotherapy, and radiation therapy, with cell therapy becoming a focal point of basic and clinical research in recent years.

In May 2020, Professor Zhai Bo’s team from Shanghai Jiao Tong University School of Medicine’s Renji Hospital, in collaboration with Shanghai Sci-Tech Biotechnology’s team led by Li Zonghai, published groundbreaking preliminary clinical research data on CAR-T cell therapy targeting the GPC3 gene for hepatocellular carcinoma in the “Clinical Cancer Research” journal. This breakthrough study brought unprecedented hope for CAR-T cell therapy in liver cancer treatment.

Even more inspiring, their publication in the “Cancer Communications” journal showcased follow-up results of two late-stage liver cancer patients who achieved long-term tumor-free survival after receiving CAR-T cell combined with local therapy [2]. These findings shed new light on the treatment prospects for liver cancer patients.
However, despite the potential therapeutic effects of liver cancer CAR-T cell therapy, it faces challenges and obstacles. Liver cancer’s heterogeneity, tumor microenvironment, and the safety of cell therapy remain crucial issues to address.
Presently, revolutionary changes are underway in the treatment models and concepts for liver cancer. However, integrating CAR-T cell therapy into actual liver cancer treatment requires further scientific exploration and clinical research. Researchers emphasize that only through comprehensive utilization of CAR-T cells in conjunction with other treatment modalities can its therapeutic potential be fully realized.
Professor Zhai Bo’s team is currently conducting various fundamental and clinical studies aimed at exploring additional possibilities for CAR-T cell therapy in solid tumors. These studies include phase I clinical research on EpCAM CAR-T cell combined with ablative therapy for gastrointestinal tumors, phase II clinical research on Claudin18.2 CAR-T cell therapy for gastrointestinal tumors, studies on the mechanism and prevention of OTOT toxicity, among others. These endeavors will provide more experimental data and support for the application of CAR-T cells in the treatment of solid tumors.
In conclusion, liver cancer CAR-T cell therapy signifies a significant breakthrough in the field of liver cancer treatment, offering new hope for patients. Despite the challenges to overcome, the outlook for this therapy is promising and holds the potential to bring a blessing to more patients in the future.
[References]
Zhaibo, Lizonghai etc.
“Chimeric Antigen Receptor-Glypican-3 T-Cell Therapy for Advanced Hepatocellular Carcinoma: Results of Phase 1 Trials.” 《Clinical Cancer Research》, 2020.
“Combined local therapy and CAR-GPC3 T-cell therapy in advanced hepatocellular carcinoma: a proof-of-concept treatment strategy.” 《Cancer Communications》, 2023.
#HealthTech#CancerResearch #Immunotherapy #CARTcell #LiverCancer #MedicalBreakthrough #ClinicalTrials #ScienceNews #HealthcareInnovation #ResearchBreakthrough #MedicalScience #CancerTherapy #CancerAwareness #InnovativeMedicine #ImmunotherapyTreatment #ScienceUpdates #HealthcareTechnology #BiomedicalResearch #ClinicalInnovation #CancerTreatment #MedicalAdvancements #ImmunologyResearch #HealthcareIndustry #ProfessionalHealthcare
The new hope for pediatric patients with relapsed/refractory B-cell acute lymphoblastic leukemia (R/R B-ALL) boasts an overall survival rate of up to 96%.


