Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
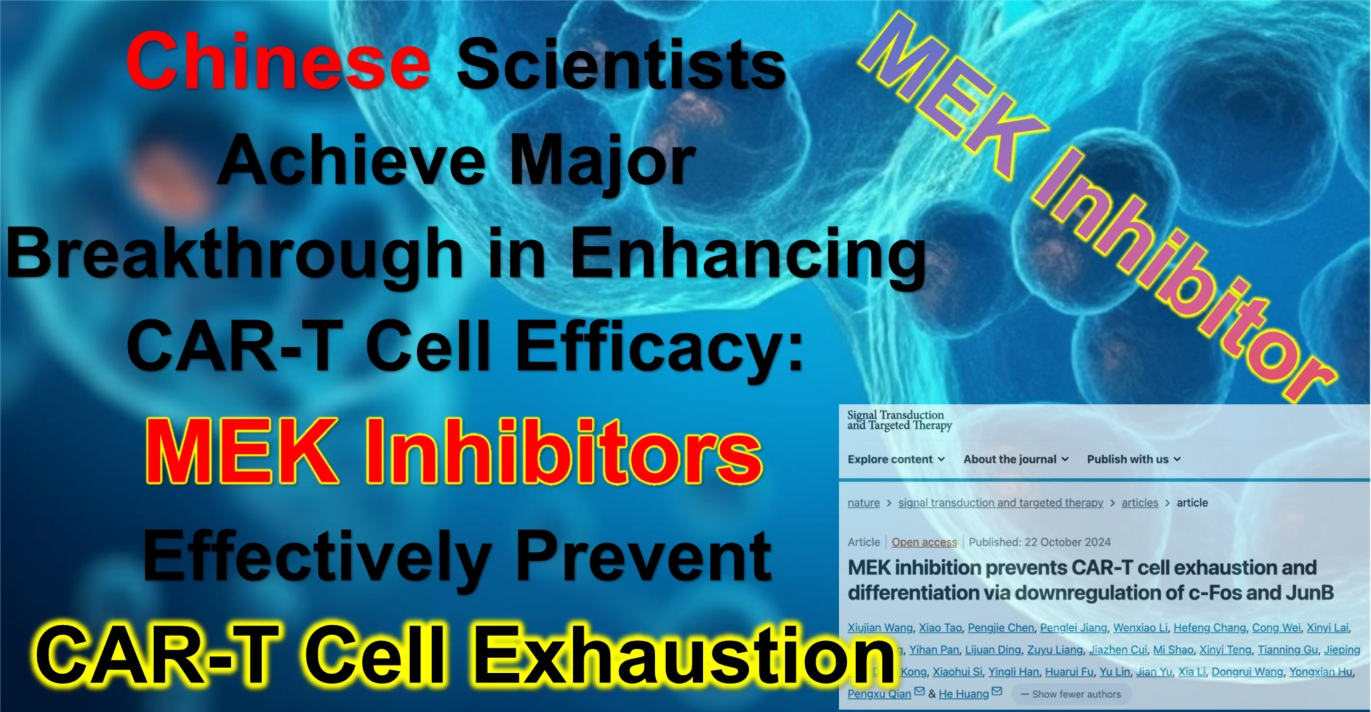
Chinese Scientists Achieve Major Breakthrough in Enhancing CAR-T Cell Efficacy: MEK Inhibitors Effectively Prevent CAR-T Cell Exhaustion
Chinese Scientists Achieve Major Breakthrough in Enhancing CAR-T Cell Efficacy: MEK Inhibitors Effectively Prevent CAR-T Cell Exhaustion

CAR-T
#CARTCellTherapy #MEKInhibitors #CancerResearch #ChineseScientists #CART #MEK
In recent years, CAR-T cell therapy has shown remarkable effectiveness in treating relapsed or refractory B-cell malignancies, particularly in cases where targeted therapies have failed. However, CAR-T therapy’s long-term effectiveness is often limited due to T-cell exhaustion and terminal differentiation, especially for patients with hematological malignancies and solid tumors. Extending the efficacy of CAR-T cells has been a global challenge in the scientific community.
On October 22, 2024, a team of Chinese medical researchers published a groundbreaking study in the prestigious journal *Signal Transduction and Targeted Therapy*, titled “MEK inhibition prevents CAR-T cell exhaustion and differentiation via downregulation of c-Fos and JunB.” This study reveals a new strategy to enhance the efficacy of CAR-T cells by using MEK inhibitors. The research demonstrates that MEK inhibitors (MEKi) can significantly reduce CAR-T cell exhaustion and terminal differentiation by suppressing the expression of c-Fos and JunB. This discovery provides a new approach to improving CAR-T therapy, especially in the context of combined treatments for both hematological malignancies and solid tumors.
MEK1/2 inhibitors have already been widely used in treating tumors with RAS or RAF mutations, playing a crucial role in cancer therapy through immune modulation. The Chinese research team further confirmed that MEKi can effectively reduce the excessive activation and exhaustion of CAR-T cells, extending their persistence and anti-tumor activity in vivo. This study not only highlights the positive impact of MEKi on CAR-T therapy but also lays the groundwork for future combination treatments to further enhance therapeutic efficacy.
The team’s research underscores the importance of the MAPK signaling pathway, particularly the roles of c-Fos and JunB in CAR-T cell function. By inhibiting the expression of these two transcription factors, the researchers successfully prevented premature exhaustion and terminal differentiation in CAR-T cells, maintaining their higher efficacy in fighting tumors.
This discovery expands the potential applications of MEKi in improving CAR-T therapy, especially through combined treatment strategies that promise to significantly improve outcomes for cancer patients. This groundbreaking study not only marks another important contribution from Chinese scientists in the field of CAR-T therapy but also opens new avenues for global cancer treatment.
Through this research, the Chinese medical research team has provided new hope for the clinical application of CAR-T therapy, revealing the potential for multiple combination treatment strategies and bringing more possibilities in the fight against cancer.
To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of Advanced Medicine in China for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CancerBreakthrough #Immunotherapy #TargetedTherapy #TCellExhaustion #CARTInnovation #CancerTreatment #MedicalResearch #Oncology #Biotechnology
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese Medical Team: Long Survival and Significant Benefits with BCMA CAR-T Treatment for RRMM: A 5-Year Outlook
**Chinese Medical Team: Long Survival and Significant Benefits with BCMA CAR-T Treatment for RRMM: A 5-Year Outlook**

RRMM
Chimeric antigen receptor (CAR) T-cell therapy is one of the most promising new treatments for relapsed/refractory multiple myeloma (RRMM), but reports on its long-term efficacy and safety are limited. As early as 2022, Professor Du Juan’s team from the Department of Hematology at Shanghai Changzheng Hospital published a Phase I/II study demonstrating that patients with poor physical status could also benefit from CAR-T therapy. Recently, the team updated their findings with a five-year long-term follow-up, focusing on factors affecting long-term clinical benefits. The results were published in *Clinical Cancer Research*. The following summary of the study’s content is provided by *Cancer Information* for readers’ benefit.
### Evidence for Long-Term Efficacy and Safety of BCMA CAR-T Cell Therapy
#### Patient Characteristics
The study included 49 RRMM patients who had all received at least three prior lines of therapy before undergoing BCMA CAR-T cell treatment. At enrollment, 20 patients (40.82%) had poor physical status (ECOG performance status of 3-4), 42.86% had high-risk cytogenetic features, and 63.27% had received four or more lines of treatment. At the time of infusion, 79.59% had progressive disease. Among the patients with poor physical status, 30% had extramedullary disease (EMD), 45% had high-risk cytogenetic features, 70% had received four or more lines of treatment, and 80% had progressive disease after their last line of treatment.
#### Efficacy Evaluation of BCMA CAR-T Cell Therapy HDS269B
After a median follow-up of 59.0 months, the study showed an overall response rate (ORR) of 77.55%. The ORR was similar across patients with different ECOG scores. The median progression-free survival (PFS) was 9.5 months, and the median overall survival (OS) was 20.0 months. The five-year PFS and OS rates were 21.3% and 31.4%, respectively. For patients with ECOG scores of 0-2, the median PFS was 11.0 months, compared to 4.0 months for those with scores of 3-4 (P=0.18). The median OS was 41.8 months for ECOG 0-2 patients and 10.5 months for ECOG 3-4 patients (P=0.015).
Patients who had previously undergone four or more lines of therapy had significantly shorter PFS and OS compared to those who had received fewer than four lines (PFS: P=0.012; OS: P=0.0049). Among the 11 patients with EMD at enrollment, the ORR was 64% for those with EMD and 82% for those without EMD. However, median PFS and OS were notably shorter in patients with EMD (PFS: 3.0 months vs. 10.5 months, P=0.06; OS: 5.0 months vs. 24.0 months, P=0.03).
#### MRD-Negative Status and CAR-T Cell Persistence Indicate Better Long-Term Survival
Minimal residual disease (MRD) negativity was significantly associated with longer PFS and OS. In this study, MRD data were available for 22 patients on day 28 post-infusion, with 14 patients (63.64%) achieving MRD negativity (10^-4). These patients experienced significantly longer PFS and OS compared to MRD-positive patients. Similar associations were observed with MRD status at 3 and 6 months post-infusion.
The expansion of CAR-T cells was also closely related to clinical outcomes. Patients who achieved partial response (PR) or better had higher CAR-T cell peak levels. Patients without disease progression five years post-infusion had significantly higher CAR-T cell expansion peaks than those with progression. Additionally, the duration of CAR-T cell persistence correlated with longer PFS and OS, with patients having CAR-T cells persisting for ≥6 months, ≥12 months, ≥24 months, and ≥36 months showing significantly better PFS and OS than those without detectable CAR-T cells.
#### Controlled Safety Profile of BCMA CAR-T Cell Therapy HDS269B
No new CAR-T cell-related toxicities were observed during long-term follow-up. All patients experienced at least one adverse event (AE), with the most common long-term (≥28 days post-infusion) grade ≥3 AEs being hematologic in nature. No second primary malignancies or delayed immune effector cell-associated neurotoxicity syndrome (ICANS) were observed.
This study also included survival analysis, classifying patients by PFS and OS. The results indicated that ECOG 0-2 status, fewer than four prior therapies, and CAR-T cell persistence for ≥6 months were independently associated with longer survival.
### The Potential of BCMA CAR-T Therapy and the Need for Future Optimization
Through a five-year long-term follow-up of 49 RRMM patients, this study systematically evaluated the efficacy and safety of BCMA CAR-T cell therapy HDS269B. The findings suggest that poor physical status is not a contraindication for CAR-T therapy, thus broadening the indications for this treatment. While the results are encouraging, the study has some limitations, including its open-label, single-arm design and small sample size, which, combined with the long follow-up period, could lead to some patient attrition. Furthermore, despite the lack of new severe toxicities, long-term safety requires continued observation.
Overall, this study underscores the importance of BCMA CAR-T cell therapy in the treatment of RRMM and provides a crucial basis for exploring and applying CAR-T immunotherapy in the frontline treatment of multiple myeloma.

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +86137 1795 9070
Email: doctor.huang@globecancer.com
#CAR_Therapy #BCMACART #MultipleMyeloma #CancerResearch #LongTermSurvival #MedicalInnovation #Hematology #CancerTreatment #Immunotherapy #OncologyAdvances
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

What are the adverse reactions after CAR-T cell reinfusion?
 Interpretation of Hot Topics Conerning Mutiple Myeloma Patients:
Interpretation of Hot Topics Conerning Mutiple Myeloma Patients:
 “What are the adverse reactions after CAR-T cell reinfusion?”
“What are the adverse reactions after CAR-T cell reinfusion?”
 CAR-T细胞回输后有哪些不良反应?
CAR-T细胞回输后有哪些不良反应?

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#CART #Mutiplemyeloma #carttherapy #myeloma #leukemia #drug #RRMM #cancer #cancertreatment #tcell #cell #advancedmedical #AdvancedMedicineinChina
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

🎉 #PNAS Breaking News: Revolutionary CAR-T Cell Therapy Successfully Treats Autoimmune Disease in China! 🎉
🎉 #PNAS Breaking News: Revolutionary CAR-T Cell Therapy Successfully Treats Autoimmune Disease in China! 🎉

PNAS
In a groundbreaking development, Chinese medical experts have achieved a milestone in the treatment of autoimmune diseases using CAR-T cell therapy.
🩺 A New Hope for Autoimmune Diseases
Autoimmune-mediated necrotizing myopathy (#IMNM) poses a significant challenge in clinical management due to its severe symptoms and limited efficacy of traditional pharmacological approaches. However, a ray of hope emerges with the application of Chimeric Antigen Receptor (CAR) T-cell therapy targeting B cell maturation antigen (#BCMA).
🔬 The Breakthrough Study
Published in the prestigious #journal PNAS, the study titled “Single-cell analysis of refractory anti-SRP necrotizing myopathy treated with anti-BCMA CAR-T cell therapy” by the medical team at Huazhong University of Science and Technology sheds light on a remarkable case. A patient with anti-signal recognition particle (SRP) IMNM exhibited significant improvement in clinical symptoms and sustained reduction in pathogenic autoantibodies after receiving BCMA-targeted CAR-T cell therapy for over 18 months.
🧬 Understanding the Mechanisms
Through longitudinal single-cell RNA sequencing and analysis of T cell receptors and B cell receptors, researchers elucidated the normalization of the immune microenvironment post-CAR-T cell infusion. The expansion of CD8+ CAR-T cells, coupled with dynamic phenotypic transitions in both CD4+ and CD8+ CAR-T cells, was observed, indicating a promising avenue for the treatment of autoimmune diseases.
🔍 Implications for the Future
This study not only highlights the efficacy of CAR-T cell therapy in refractory IMNM but also underscores the importance of understanding the molecular characteristics of CAR-T cells in autoimmune diseases. Further research is warranted to enhance the efficiency and durability of CAR-T cell therapy for autoimmune conditions.
🌟 Hope for Patients Worldwide
With this groundbreaking achievement, there is renewed hope for millions of individuals battling autoimmune diseases globally. Stay tuned for more updates as we continue to unlock the potential of innovative therapies in medical science!
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
Email: doctor.huang@globecancer.com,
WhatsApp: +8613717959070
#CAR_TTherapy #AutoimmuneDisease #MedicalBreakthrough #Science #Innovation #PNAS #cart #cartcell #BCMA #IMNM #chineseresearch #medicalscience
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Miracle Unfolds: Zhejiang University Hospital’s Custom CRISPR/Cas9 Gene Editing + CAR-T Cell Therapy Rebirths Patients
🌟 Miracle Unfolds: Zhejiang University Hospital’s Custom CRISPR/Cas9 Gene Editing + CAR-T Cell Therapy Rebirths Patients 🌟
👨👧👦 (“I want to live to see my child go to university!”)👨👧👦

CAR-T Cell Therapy Patient
🔬14 rounds of chemotherapy, 20 rounds of radiotherapy, total gastrectomy… For two years,
🍋Seeking a glimmer of hope, he turned to Zhejiang University Hospital in Hangzhou. This time, the doctors said, “Let’s give it a try together.”
💪The brand-new PD1-19bbz CAR-T cells, with a low risk of cytokine storms and other complications, gave Mr. Sun a new lease on life.
🏸Today, Mr. Sun has been disease-free for over 2 years.

Tsinghua University
Email: doctor.huang@globecancer.com,
WhatsApp: +8613717959070
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
2024 Lancet: Revolutionizing Cancer Treatment: China’s Breakthroughs in CAR T-Cell Therapy
🌟2024 Lancet: Revolutionizing Cancer Treatment: China’s Breakthroughs in CAR T-Cell Therapy 🌏

LANCET
Dive into the cutting-edge world of CAR T-cell therapy, where China is making waves in the realm of cellular treatments. Since the inception of CAR T-cell clinical trials in 2013, this groundbreaking therapy has become a beacon of hope for cancer patients across the country.
🔬 Explosive Growth: By 2017, China led the globe in the number of CAR T-cell-related clinical trials, marking a pivotal moment in the evolution of cancer treatments. Fast forward to 2021, and Chinese cell therapy companies have amassed a staggering $237 million in funding, reflecting the robust expansion of CAR T-cell therapy in the nation.
🌐 Government Support: A deep dive into the research unveils the influential role of Chinese government policies in propelling CAR T-cell therapy forward. Strong governmental backing, coupled with capital influx, massive patient demand, and a unique healthcare system, lays the foundation for the accelerated growth of this revolutionary therapy in China.
🎗️ Overcoming Challenges: While CAR T-cell therapy is still in its infancy for solid tumors, it has achieved remarkable success in treating blood cancers. China has emerged as a global leader in conducting clinical trials, particularly focusing on hematologic malignancies like B-cell lymphomas. CAR-T cells, reprogrammed to combat cancer, offer a beacon of hope for those facing these formidable diseases.
🚀 Pushing Boundaries: Beyond blood cancers, Chinese researchers are actively exploring the potential of CAR-T cell therapy in various solid tumors. Preliminary studies hint at promising results for liver, pancreatic, and brain cancers. China’s commitment to medical innovation shines through as it pushes the boundaries of cancer research and treatment strategies.
🌈 Hope for the Future: Join us in celebrating the strides China is making in cancer research. Every breakthrough in CAR T-cell therapy brings us closer to a future where cancer is not just treated but conquered.
💪🏼🔬 #CARTCellChina #MedicalInnovation #CancerBreakthrough #HopeForTheFuture #GlobalHealthRevolution #CellTherapy #LANCET #HEMATOLOGY
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
Breakthrough CAR-T Cell Therapy for R/R B-Cell ALL: A Game-Changer in Chinese Medical Innovation


