Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

New Advances in Chinese CAR-T Cell Therapy: CD27 Armored BCMA CAR-T Shows Potential in Treating Relapsed Multiple Myeloma
**New Advances in Chinese CAR-T Cell Therapy: CD27 Armored BCMA CAR-T Shows Potential in Treating Relapsed Multiple Myeloma**

Multiple Myeloma
#BCMA #CD27#CARTCellTherapy #RRMM #MM #MultipleMyeloma #CART
Relapsed and refractory multiple myeloma (RRMM) is a challenging blood cancer that poses significant obstacles for patients. Although several BCMA (B-cell maturation antigen) targeting CAR-T cell therapies have been developed, achieving long-term remission and extended survival remains difficult. The recent introduction of CD27-armored BCMA CAR-T—CBG-002—by a Chinese medical team brings new hope for RRMM patients. Based on preclinical data, this therapy shows promising anti-myeloma activity, with Phase I clinical trial results further validating its efficacy and safety.
Research Highlights
CBG-002 is an innovative therapy that enhances traditional BCMA CAR-T by adding a CD27 costimulatory domain. The inclusion of CD27 improves CAR-T cell persistence and activity, contributing to stronger anti-cancer effects. Recently published Phase I research in Cancer Immunology Research indicates that this CD27-armored BCMA CAR-T therapy is not only well-tolerated but also shows notable efficacy in RRMM patients.
The study enrolled 11 RRMM patients, all of whom had undergone three or more prior treatments, with a median age of 54. The results showed that 81.8% of patients experienced only mild cytokine release syndrome (CRS) and no severe neurotoxicity. Even at a low dosage level (1-3×10^6 CAR-T cells/kg), CBG-002 achieved a high overall response rate (ORR) of 81.8%, with 45.5% of patients reaching stringent complete remission.
Future Outlook
The Phase I study of CBG-002 not only confirmed its efficacy but also demonstrated advantages in production time and cost. Compared to current products on the market, CBG-002 has a shortened production cycle of just 10 days, making it a timely and affordable treatment option for more patients. Amid global progress in CAR-T cell therapies, the innovation by this Chinese medical team positions it as a significant force in advancing cancer research worldwide.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#MultipleMyeloma #CancerResearch #Immunotherapy #ChinaBiotech #Oncology
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

CAR-T Cell Therapy as a New Option for Treating Multiple Myeloma
**CAR-T Cell Therapy as a New Option for Treating Multiple Myeloma**

Multiple Myeloma
“CAR-T cell therapy has now become a new option for treating patients with relapsed and refractory multiple myeloma, with hopes of changing the difficult situation in myeloma treatment where treatments are either ineffective or unavailable,” said Jin Jie, Director of the Hematology Department at the First Affiliated Hospital of Zhejiang University School of Medicine, in an interview with People’s Daily Health on October 23.
Early on October 23, Jin Jie visited the hospital ward to see a 72-year-old patient with multiple myeloma. Just the day before, the patient had begun receiving treatment with a domestically produced BCMA-targeting CAR-T drug.
“This 72-year-old patient came to the hospital due to bone pain and was even unable to walk steadily,” Jin recalled. After being diagnosed with multiple myeloma, the patient initially received a series of chemotherapy regimens that showed some efficacy. However, a year after undergoing autologous hematopoietic stem cell transplantation, the disease relapsed, and various subsequent treatments proved suboptimal. As a result, the patient opted for CAR-T therapy.
Since the approval of Equecabtagene Autoleucel last year, Professor Jin Jie became one of the first doctors nationwide to prescribe CAR-T drugs for multiple myeloma. This patient is her tenth multiple myeloma patient to receive this innovative therapy. Discussing the treatment outcomes, Jin noted, “The overall efficacy for patients has been very good.” A month ago, another patient who underwent this CAR-T therapy returned for a follow-up. “Everything was going well; we will continue to monitor the patient’s indicators until all data are stable.”
Multiple myeloma is a malignant plasma cell disease that predominantly affects older adults. Jin observed that the incidence of multiple myeloma has been trending younger in recent years, with patients in their forties commonly seen in clinical settings. Currently, multiple myeloma remains an incurable disease, and most patients face inevitable relapse. However, thanks to the emergence of new drugs and advances in treatment, both the efficacy and overall survival of patients have significantly improved in recent years.
“CAR-T therapy involves collecting a patient’s T cells and equipping them with a ‘weapon.’ These T cells are then expanded outside the body and re-infused into the patient, where they target and attack tumor cells, while also being able to proliferate in the patient’s body to eliminate cancer cells,” Jin explained. CAR-T therapy has the potential to give patients a higher quality of life.
CAR-T cell therapy has been advancing rapidly in the field of multiple myeloma, with multiple products already approved and available. In Jin’s view, patients should, under professional guidance, choose drugs and treatments with extensive clinical experience and proven efficacy.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
**#MultipleMyeloma #CAR_TCellTherapy #CancerTreatment #Immunotherapy #Hematology #CellTherapy #MedicalInnovation #PatientCare #Oncology #ZhejiangUniversity**
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China’s CAR-T Cell Therapy: Leading a New Revolution in the Treatment of Relapsed/Refractory Mantle Cell Lymphoma (MCL)

MCL
#CARTCellTherapy #MantleCellLymphoma #RelapsedMCL #RefractoryMCL #Hematology #MCL
Mantle Cell Lymphoma (MCL), a rare and complex subtype of non-Hodgkin’s lymphoma, exhibits both aggressive and indolent characteristics. Since MCL patients are usually diagnosed at an advanced stage, often with poor prognostic factors, traditional treatments such as chemotherapy and immunotherapy have brought survival benefits to some patients. However, most still face disease relapse or progression, particularly those with relapsed/refractory (r/r) MCL, where treatment poses significant challenges. For a long time, treatment options for MCL have been limited, making it difficult to achieve long-term remission or cure, highlighting the need for breakthroughs.
Chinese CAR-T Cell Therapy: Offering Hope for Relapsed/Refractory MCL With the rapid development of biotherapeutics, Chinese CAR-T cell therapy has emerged as a breakthrough innovation in lymphoma treatment. Since the approval of the world’s first CAR-T product in 2017, China has made significant progress in CAR-T research and application, especially in treating r/r MCL, where CAR-T therapy has shown excellent efficacy. CAR-T therapy not only significantly improves patient remission rates but also offers new survival opportunities for high-risk patients with poor prognoses.
Efficacy: In recent years, multiple international and Chinese clinical studies have confirmed the efficacy of CAR-T cell therapy in patients with relapsed/refractory MCL. Clinical data from China have also demonstrated outstanding results. In August 2024, the Chinese CAR-T product Relmacabtagene Autoleucel, a CD19-targeted CAR-T cell therapy, was approved for its third indication, treating adult patients with relapsed/refractory MCL (r/r MCL). Clinical data showed that Relmacabtagene Autoleucel achieved an overall response rate of 81.4%, with a complete response rate of 67.8%. The median progression-free survival (PFS) was 13 months, and the median overall survival (OS) was 19.5 months. These data further prove that Chinese CAR-T cell therapy not only has high efficacy in r/r MCL patients but also provides lasting survival benefits.
Side Effect Management: Despite its remarkable efficacy, CAR-T therapy is associated with certain side effects, such as cytokine release syndrome (CRS) and neurotoxicity (NT). However, with the continuous optimization of treatment technologies and accumulated experience in China, the incidence of side effects has been effectively controlled. In treating relapsed/refractory MCL patients, Chinese medical teams have been able to limit the occurrence of grade ≥3 CRS and NT to 6.8% in clinical practice. This lower toxicity makes CAR-T therapy safer in clinical applications, offering a new option for patients who cannot tolerate traditional treatments. Particularly for those who are resistant to Bruton’s tyrosine kinase inhibitors (BTKi) or ineligible for autologous hematopoietic stem cell transplantation, Chinese CAR-T therapy provides a gentler and effective treatment option.
Indications: The following groups of patients can benefit from Chinese CAR-T cell therapy:
-
Patients with relapsed/refractory MCL (r/r MCL)
-
MCL patients who do not respond to conventional treatments
-
Patients with poor prognostic factors (TP53 mutation, Ki67≥30%, blastoid MCL, pleomorphic MCL, POD24)
-
Patients previously exposed to BTKi or resistant/relapsed/refractory MCL
-
High-risk patients unsuitable for transplantation who cannot achieve good remission
Conclusion: With the widespread application of Chinese CAR-T therapy globally, more MCL patients, both domestic and international, are able to achieve deep remission and long-term survival. This not only marks a significant advancement in China’s hematological cancer treatment but also signifies that Chinese CAR-T cell therapy is leading a new transformation in the treatment of relapsed/refractory lymphoma worldwide.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CancerTreatment #Immunotherapy #CancerResearch #Chinesemedicalbreakthrough #LymphomaTreatment #Oncology #Biotherapy #CancerInnovation
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Why Are More and More International Patients Coming to China for CAR-T Cell Therapy?
Why Are More and More International Patients Coming to China for CAR-T Cell Therapy?

CARTtherapy
#CARTtherapy #InternationalPatient #CART #Multiplemyeloma #lymphoma #leukemia
In recent years, CAR-T cell therapy, as an innovative cancer treatment, has rapidly gained widespread attention globally. China, as a leading country in the CAR-T treatment field, has attracted an increasing number of patients from Europe and Asia due to its advanced technology, high-quality medical resources, and relatively lower treatment costs. So, why are more international patients choosing to come to China for CAR-T treatment? Here are some key reasons worth noting:
### 1. **Advanced CAR-T Treatment Technology**
Over the past decade, China has made tremendous strides in medical research, particularly in the field of cancer immunotherapy. Not only has China successfully developed various CAR-T therapies, but it has also built a comprehensive treatment protocol and safety monitoring system to ensure efficient and safe treatment for patients. Additionally, China has accumulated extensive experience in managing treatment-related side effects, such as CRS (Cytokine Release Syndrome) and neurotoxicity.
Currently, more than 50% of the world’s clinical trials for CAR-T therapy are conducted in China. Of the 11 CAR-T products already on the global market, five are from China. These include products for multiple myeloma (BCMA CAR-T), diffuse large B-cell lymphoma (CD19 CAR-T), and leukemia. Compared to similar products in the U.S. for multiple myeloma, China’s CAR-T products are not only fully humanized but also boast the highest complete remission rates among all available products, reaching 82.4%. The CR data for China’s lymphoma and leukemia CAR-T products are also impressive, at 77.6% and 82.1%, respectively.
### 2. **International Certification and Clinical Trial Opportunities**
Several hospitals and research institutions in China have gained international recognition for their CAR-T therapies. Patients can choose between commercial CAR-T treatments or participate in clinical trials. For some CAR-T treatments that have not yet been approved or are hard to access in Europe or Asia, patients can receive these cutting-edge therapies in China earlier.
Just a few days ago, Chinese scholars’ research on universal CAR-T cell therapy was featured on the cover of *Nature*. Various other studies on Chinese CAR-T therapies are frequently published in major medical journals. For example, a Chinese medical team published a study on dual-target CAR-T in *JAMA Oncology*, showing a 100% stringent complete remission (sCR) rate in high-risk multiple myeloma patients. Additionally, a research team from Zhejiang, China, published clinical data in *Cell Discovery*, highlighting the breakthrough of the fourth-generation anti-CD19 CAR-T cell therapy in treating relapsed/refractory large B-cell lymphoma with exceptional anti-tumor capabilities.
### 3. **Relatively Low Treatment Costs**
Compared to the million-dollar treatment costs in Western countries, CAR-T treatment in China is relatively affordable, often costing just 1/5 to 1/7 of the price in the U.S. For instance, the cheapest CAR-T product for multiple myeloma in the U.S. costs $420,000, and when including hospitalization and other expenses, the total cost can reach around $700,000. In contrast, similar Chinese products, with equal or even better effectiveness, cost only $160,000, and hospitalization and other fees are much lower, often under $10,000. Some treatment options are available for just a few tens of thousands of dollars, and in certain cases, treatment is even completely free.
Patients from Europe and Asia can receive world-class CAR-T therapy in China at a much lower cost, providing an affordable option for those who find the high costs of treatment elsewhere prohibitive. Moreover, a range of one-stop medical services, including hospital visits, translation assistance, transportation, accommodation, and even family care, can significantly enhance the patient experience for international patients.
### 4. **World-Class Expert Teams**
China is home to a group of internationally renowned oncology and immunotherapy experts who have extensive clinical experience and academic achievements in hematological cancers and CAR-T therapy. Patients receive personalized treatment plans designed by leading experts, ensuring that each treatment is precisely tailored to their specific condition, maximizing effectiveness and minimizing risks.
For example, Professor Huang Xiaojun, Director of the Institute of Hematology at Peking University, is one of the world’s pioneers in haploidentical transplantation, having developed China’s haploidentical hematopoietic stem cell transplantation model. His innovative approach has increased the 3-year survival rate of leukemia patients receiving haploidentical transplants from around 20% to approximately 70%, earning him the Distinguished Service Award from the Center for International Blood and Marrow Transplant Research.
### 5. **Efficient and Convenient Medical Services**
China has implemented a 144-hour visa-free transit policy for citizens of 54 countries and mutual visa exemptions with 24 countries. This is a major benefit for international patients seeking treatment in China. Moreover, China’s healthcare system is known for its efficiency and convenience, especially in treating major diseases. Appointment wait times are short, and there is minimal delay in receiving treatment. A “super green channel” is also available for international patients, ensuring quick diagnosis and treatment planning. From initial consultation to online meetings with specialists, the process typically takes less than a week, with hospital admission and treatment beginning within another week.
Compared to the long waiting periods in other countries, China’s efficient medical services significantly improve treatment success rates and patient satisfaction. CAR-T therapy, in particular, is known for its high efficiency. There was an international patient who went from initial consultation to complete remission and a safe return home in less than a month—a remarkable achievement considering CAR-T is a “living” drug that must be custom-prepared using the patient’s own blood cells.
### 6. **Abundant Success Stories**
Many international patients who have received CAR-T therapy in China have achieved remarkable results, with their cancers being effectively controlled or even cured. These success stories not only boost confidence among patients worldwide but also attract more people to China for treatment. By sharing these stories, more patients learn about China’s advantages in CAR-T therapy and choose it as their treatment destination.
For instance, Ethan, a patient from Singapore, achieved complete remission just four weeks after CAR-T treatment and has since recovered very well. There is also the story of a Russian patient with high-risk, late-stage multiple myeloma who received efficient treatment in China at a much more reasonable cost and with a faster treatment process.
### 7. **Conclusion**
In conclusion, as China continues to enhance its technological capabilities in CAR-T cell therapy, more and more patients from around the world are choosing to come to China for this cutting-edge cancer treatment. With its advanced medical technology, international services, and relatively lower treatment costs, China is becoming an important destination for cancer patients seeking treatment. If you or a loved one is searching for innovative cancer treatment options, consider CAR-T therapy in China for a chance at recovery!
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can contact us for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CellTherapy #CancerTreatment #Immunotherapy #HealthcareInnovation #MedicalTourism #ChinaHealthcare #CancerSurvivor #PatientStories #MedicalAdvancements #AffordableHealthcare #PersonalizedMedicine #GlobalHealth #HealthJourney #Oncology
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Why More and More Foreigners Are Choosing China for CAR-T Cell Therapy
**Why More and More Foreigners Are Choosing China for CAR-T Cell Therapy**

CAR-T
In recent years, China’s advancements in cell therapy, particularly CAR-T therapy, have been remarkable, attracting numerous international patients seeking treatment. With its cutting-edge technology, reasonable costs, and outstanding efficacy, China has become a preferred destination for cancer patients worldwide. Today, we will explore why an increasing number of foreign patients choose to come to China for cell therapy.
**The Rise of CAR-T Therapy: China’s Advantages**
First, CAR-T therapy is a personalized immunotherapy that specifically targets blood-related cancers such as lymphoma, multiple myeloma, and leukemia. By engineering a patient’s own T-cells to recognize and attack cancer cells, CAR-T therapy has shown significant efficacy, especially in patients with advanced cancer.
China’s advantages in cell therapy can be summarized into three key points:
-
**Technological Expertise**: China has accumulated extensive experience in CAR-T therapy, particularly in top hospitals in major cities like Shanghai and Beijing. These institutions boast world-class medical teams and advanced treatment systems, providing international patients with high-quality treatment plans that ensure global leading outcomes.
-
**Affordable Costs**: While CAR-T therapy can cost millions of dollars in countries like the U.S. and Europe, the treatment in China is much more affordable, usually about one-seventh to one-tenth of the cost in the U.S. This affordability makes China an attractive option for many international patients.
-
**Shorter Waiting Times**: In Western countries, patients often wait several months for CAR-T therapy, whereas in China, many hospitals can complete the entire process — from diagnosis to cell infusion — in just a few weeks. This is crucial for patients with urgent medical needs.
**Real-Life Cases: Why Foreign Patients Choose China**
Several real-life cases clearly demonstrate why international patients are increasingly seeking treatment in China.
– **Ethan from Singapore**
Ethan had been battling cancer for nearly ten years, trying various treatments globally with little success. Eventually, he decided to seek treatment in China. After a thorough evaluation at Jiahui International Hospital in Shanghai, Ethan received a personalized CAR-T therapy plan. Within just four weeks, his cancer cells had significantly decreased, and his health began to improve, along with his weight. Ethan remarked, “The treatment in China is not only effective but also much more affordable than in Singapore.”
– **A Russian Patient’s Story**
Borzinkov, a 70-year-old patient with high-risk advanced multiple myeloma, had experienced multiple treatment failures before learning about China’s CAR-T therapy. He traveled to China for treatment, successfully completed cell collection, and received CAR-T infusion. Just 13 days later, he was discharged, and his follow-up results have been very promising. He chose China not only for the lower costs but also because of the shorter waiting time.
These cases illustrate that international patients in China can receive high-quality, effective treatment at more affordable rates and with faster recovery times. So far, patients from countries like Russia, Singapore, Malaysia, and others have undergone CAR-T therapy in China and achieved significant results, with many experiencing complete remission within months, with no signs of cancer after treatment.
**China’s Leading Position and Future Prospects**
China has already established itself as a global leader in CAR-T therapy, accounting for over 50% of the world’s clinical trials in this field. Chinese researchers and doctors excel not only in the basic research and clinical applications of CAR-T therapy but also in managing side effects such as CRS and neurotoxicity.
In cities like Shanghai, Wuhan, and Beijing, doctors provide cutting-edge treatment to international patients daily, consistently delivering excellent outcomes. As China’s medical system continues to advance, more and more international patients are choosing China as their destination for treatment.
**Conclusion**
The reasons why foreign patients are choosing China for cell therapy are clear: China excels in technology, offers reasonable treatment costs, and delivers outstanding results. As the world becomes more aware of the progress in China’s healthcare system, more patients will seek advanced medical care in China. In particular, China’s breakthroughs in cell therapy will continue to attract cancer patients from across the globe.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can contact us for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CARtherapy #CellTherapy #CancerTreatment #Immunotherapy #HealthcareInnovation #MedicalTourism #ChinaHealthcare #CancerSurvivor #PatientStories #MedicalAdvancements #AffordableHealthcare #PersonalizedMedicine #GlobalHealth #HealthJourney #Oncology #CAR-T
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
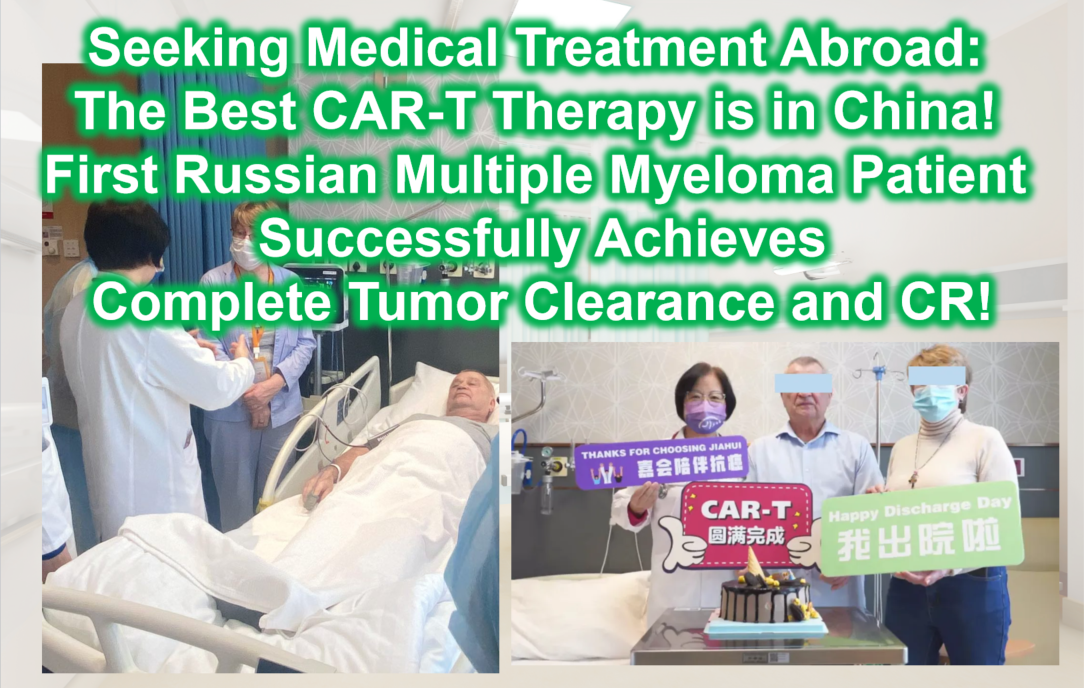
Seeking Medical Treatment Abroad: The Best CAR-T Therapy is in China! The First Russian Multiple Myeloma Patient Successfully Achieves Complete Tumor Clearance and CR!
**Seeking Medical Treatment Abroad: The Best CAR-T Therapy is in China! The First Russian Multiple Myeloma Patient Successfully Achieves Complete Tumor Clearance and CR!**

patient story
Over the five years following his diagnosis of high-risk multiple myeloma, Mr. F endured relentless pain. Despite undergoing various chemotherapy regimens and two transplants, he could never completely escape the nightmare of unpredictable tumor recurrence and progression. Fortunately, by the end of 2023, his condition temporarily stabilized. Accompanied by top hematology experts from Russia, Mr. F traveled to China to receive CAR-T therapy, achieving optimal results with complete tumor eradication and CR (complete remission).
Achieved CR! In February 2024, Mr. F and his family successfully returned to Russia to resume their happy lives.
(Note: To protect patient privacy, pseudonyms are used in the text, and the medical details and treatment process have been slightly modified.)

**Common Back Pain Turned Out to Be High-Risk Multiple Myeloma**
Mr. F, 63, is the vice principal of a technical college in St. Petersburg, Russia. He has a successful career and a happy family with his wife, one son, and two daughters. In the months leading up to Christmas 2017, Mr. F experienced worsening back pain, with severe pain in the lumbar region. Accompanied by his family, he went to the hospital for a thorough examination. The final diagnosis was multiple myeloma, with osteolytic lesions in more than three areas. The doctor informed Mr. F that, based on the comprehensive assessment of various test results, his myeloma was classified as high-risk (IgG-type, DS-Stage IIIA, ISS-Stage II), making it difficult to treat, prone to relapse, and with a poor prognosis. This devastating news struck suddenly, plunging the entire family into shock and panic. In an interview, Mr. F said, “In Chinese culture, Confucius said, ‘When Heaven is about to place a great responsibility on a man, it always first frustrates his spirit,’ viewing it as a life test. In Western culture, it is seen as God’s punishment, a trial one must endure, with the outcome entirely dependent on how one faces these challenges. I must choose to fight!”
**Relapse and Progression: The Unshakeable Nightmare for Multiple Myeloma Patients**
After two days of shock and panic, Mr. F decided to pull himself together and seek the best treatment to fight the tumor. Mr. F and his family consulted many doctors and clinics in Spain, Munich, and Heidelberg. Upon learning that CAR-T therapy had remarkable curative effects on hematologic malignancies, they felt a glimmer of hope, which was soon extinguished. At that time, very few countries offered CAR-T therapy, mostly in developed nations, and the exorbitant cost of nearly a million dollars made it unaffordable even for the well-off Mr. F. To control the disease as quickly as possible, Mr. F underwent traditional CVD therapy in Russia at the beginning of 2018, followed by his first autologous bone marrow transplant, achieving partial remission (PR). Unfortunately, just ten months later, his multiple myeloma relapsed. After switching to a second-line chemotherapy regimen, he underwent a second autologous bone marrow transplant. Despite receiving the most aggressive treatment and enduring life-threatening side effects, tumor lesions persisted in his body, like a ticking time bomb that could explode at any moment. Although maintenance therapy temporarily stabilized the disease, Mr. F and his family remained vigilant. They knew that high-risk myeloma was likely to relapse and progress again in the short term. If the disease worsened once more, they would face a desperate situation with no available treatments.

**Global Comparison Shows the Best CAR-T Therapy is in China!**
To completely rid himself of cancer and escape the nightmare of constant relapse and deterioration, five years later, Mr. F and his family once again sought the possibility of a cure through CAR-T therapy worldwide. They conducted extensive research on the CAR-T therapies already on the market and consulted numerous hospitals and experts in Europe, including in India and Israel. To his surprise, despite five years passing, there had been no significant progress in these countries. Almost every expert mentioned that Chinese hospitals had made tremendous advancements in the CAR-T field, surpassing even those in Europe and the United States. Especially for multiple myeloma, on June 30, 2023, China introduced the world’s first fully human BCMA CAR-T therapy, Iquilence. Mr. F and his attending physician immediately contacted Shanghai Jiahui International Hospital in China for an initial remote consultation. Jiahui Hospital, an internationally aligned oncology center with a professional multidisciplinary MDT team, conducted a preliminary evaluation. They concluded that the Iquilence (BCMA CAR-T) treatment plan would be most beneficial and developed a comprehensive plan, addressing all questions and concerns of Mr. F and the Russian experts, ultimately reaching a consensus on the treatment plan.
Professor Li Hua, Director of Oncology at Jiahui International Cancer Center, and his team of Russian hematology experts discussed Mr. F’s treatment plan. Although Mr. F was somewhat aware of China’s medical advancements, he couldn’t imagine that China’s healthcare had surpassed that of the developed Western countries. Therefore, in November 2023, Mr. F’s attending physician, a renowned Russian hematology expert, accompanied him to China to evaluate the feasibility of Chinese CAR-T therapy on-site. Their first stop upon arriving in China was to meet with Jiahui Hospital’s international multidisciplinary treatment team and tour the medical environment that adheres to international nursing standards.

Professor Li Hua, Director of Jiahui International Cancer Center, and his team provided a detailed introduction to the CAR-T treatment plan. Compared to traditional treatments, CAR-T has unique advantages. Firstly, it is a highly personalized treatment that adjusts the patient’s immune system through genetic engineering, providing a more precise response to cancer treatment. Secondly, CAR-T shows significantly higher efficacy in complex cases, which traditional treatments cannot achieve. Iquilence has an overall response rate of nearly 99% and a complete response rate of up to 82.4%. More importantly, fully human CAR-T not only maintains the patient’s quality of life but also provides more durable treatment effects. Additionally, a one-time infusion treatment achieves rapid and effective results, reducing the pain and burden during the treatment process and bringing hope for a cure.+

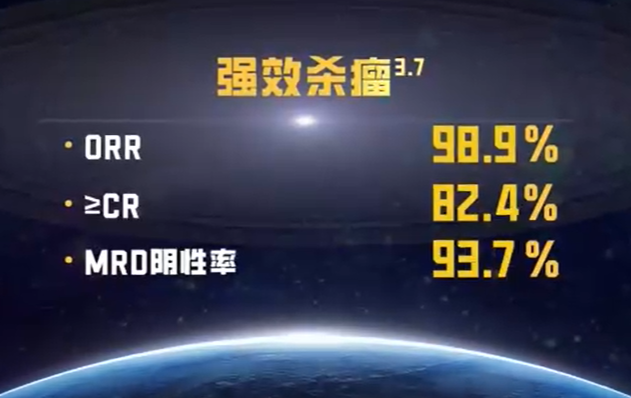
The second stop for Mr. F and the Russian experts was a visit to the production facility of the world’s first fully human BCMA CAR-T therapy, Iquilence. Mr. F and the Russian experts were impressed to learn that this exceptional therapy was not only entirely developed in-house, but the lentiviral vector was also independently developed. The facility boasts a complete end-to-end development platform and a self-built manufacturing base.

Finally, Mr. F overcame all his concerns and was convinced that China’s fully human BCMA CAR-T solution was world-leading. He decided to undergo treatment in China.
All Tumors Cleared for the First Time, Advanced Myeloma Achieves CR!
In December 2023, just before another Christmas five years after his diagnosis, Mr. F and his family arrived in China, full of hope for a cure, to officially begin his CAR-T journey. At Shanghai Jiahui International Hospital, Mr. F underwent thorough examinations, and after meeting all the requirements, he successfully underwent apheresis, the process of collecting T cells. These cells were then transported back to the factory within 24 hours to create a precisely targeted anti-cancer weapon tailored for Mr. F.
On January 20, 2024, he received lymphodepleting chemotherapy with fludarabine and cyclophosphamide.
On January 25, 2024, the CAR-T cells, carrying the hopes of Mr. F’s entire family, were transported to him by the pharmacy team using professional cold chain logistics. After multiple inspections, unpacking, and reactivation, a small bag of milky white liquid was infused back into Mr. F. The doctors explained that this liquid contained hundreds of billions of “special forces” T cells, which, once in the body, would begin an intense sweep to eradicate the myeloma cells completely.
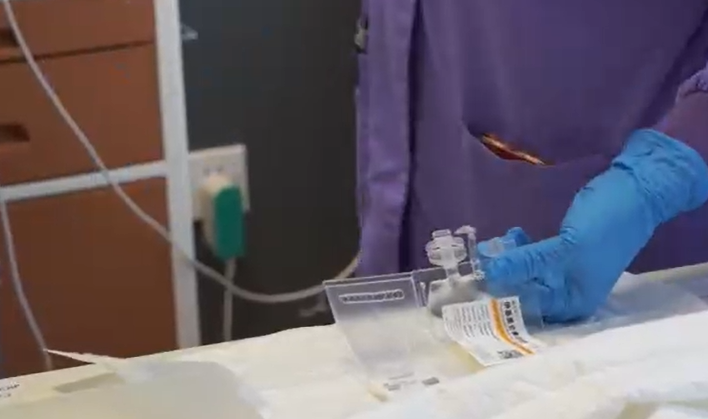
The infusion process went smoothly. What deeply moved Mr. F and his family was that whenever he felt anxious, the medical team would hold his hand, giving him confidence and strength.
Although a mild cytokine release syndrome (CRS), which is common after CAR-T therapy, occurred post-infusion, the medical team at Jiahui Hospital was very experienced in handling this. With symptomatic treatment, the symptoms improved in about a week. On the 14th day after the infusion, the evaluation showed that the CAR-T cells had expanded effectively in Mr. F’s body, reaching 10^9.
Mr. F said that, unlike the two painful transplant experiences, despite knowing that CAR-T therapy has side effects, the medical team clearly informed him about the reactions he might have at different stages and assured him that the medical staff would address them immediately. As a result, he felt no concerns or fears.
Before the Chinese New Year in 2024, Mr. F was discharged and returned to Russia for further follow-up in his home country. Professor Li Hua said, “The treatment results met our expectations, with significant improvements in key indicators such as serum M protein, free light chains, and disease symptoms. All functional statuses have improved.”
In May 2024, the PET-CT results showed that Mr. F’s lesions had disappeared, and he finally achieved complete remission (CR). This means that after battling multiple myeloma for six years, he has finally won!
A Brighter Future: China Will Become the Best Treatment Destination for Advanced Myeloma Patients!
Now, Mr. F not only resumes his happy and fulfilling life, but he and his wife have also returned to their beloved careers. He currently serves as the General Manager of the UNESCO Mineral Resources Education Center.
Mr. F said, “When I came to China and saw the medical conditions and experienced the best care firsthand, I felt a deep sense of pride for China. It is regrettable that Russia is currently unable to achieve this level of care. However, I will bring my experiences here back to Russia and share them. China’s medical system is world-leading, and choosing to come to China was the right decision!”

In an interview, Mr. F’s wife said, “Although we are past retirement age, we both have very important careers. This CAR-T treatment in China has given us a very precious experience. We have more time to listen to each other’s thoughts, support each other, and get through difficult times together.”

Professor Li Hua, Director of the International Oncology Hospital and Director of the Department of Oncology, said in an interview:
“Clinical research on CAR-T therapy started relatively early in China. Especially since 2020, the number of CAR-T clinical trials in China has far surpassed that in the United States, giving us extensive experience from laboratory research to clinical application.
Moreover, CAR-T treatment in China is more cost-effective, providing patients with a more competitive option.
Most importantly, China has sufficient commercial-quality production capacity. Patients can receive treatment quickly without waiting, which is crucial for those with malignant hematologic diseases who need timely treatment.”
Professor Li Hua, Director of Oncology at Jiahui International Hospital,
The emergence of CAR-T cell therapy has brought new hope to cancer patients worldwide. China’s CAR-T therapy offers outstanding efficacy, good safety, low cost, and rapid preparation advantages. Several international hospitals have opened green channels for overseas patients, making China the best treatment destination for global late-stage multiple myeloma patients!

References:
[1] Yuting Yan, et al. Blood Adv. 2019 Oct 8;3(19):2895-2904.[2] 2023 IMS. P-290.
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese Medical Team: Long Survival and Significant Benefits with BCMA CAR-T Treatment for RRMM: A 5-Year Outlook
**Chinese Medical Team: Long Survival and Significant Benefits with BCMA CAR-T Treatment for RRMM: A 5-Year Outlook**

RRMM
Chimeric antigen receptor (CAR) T-cell therapy is one of the most promising new treatments for relapsed/refractory multiple myeloma (RRMM), but reports on its long-term efficacy and safety are limited. As early as 2022, Professor Du Juan’s team from the Department of Hematology at Shanghai Changzheng Hospital published a Phase I/II study demonstrating that patients with poor physical status could also benefit from CAR-T therapy. Recently, the team updated their findings with a five-year long-term follow-up, focusing on factors affecting long-term clinical benefits. The results were published in *Clinical Cancer Research*. The following summary of the study’s content is provided by *Cancer Information* for readers’ benefit.
### Evidence for Long-Term Efficacy and Safety of BCMA CAR-T Cell Therapy
#### Patient Characteristics
The study included 49 RRMM patients who had all received at least three prior lines of therapy before undergoing BCMA CAR-T cell treatment. At enrollment, 20 patients (40.82%) had poor physical status (ECOG performance status of 3-4), 42.86% had high-risk cytogenetic features, and 63.27% had received four or more lines of treatment. At the time of infusion, 79.59% had progressive disease. Among the patients with poor physical status, 30% had extramedullary disease (EMD), 45% had high-risk cytogenetic features, 70% had received four or more lines of treatment, and 80% had progressive disease after their last line of treatment.
#### Efficacy Evaluation of BCMA CAR-T Cell Therapy HDS269B
After a median follow-up of 59.0 months, the study showed an overall response rate (ORR) of 77.55%. The ORR was similar across patients with different ECOG scores. The median progression-free survival (PFS) was 9.5 months, and the median overall survival (OS) was 20.0 months. The five-year PFS and OS rates were 21.3% and 31.4%, respectively. For patients with ECOG scores of 0-2, the median PFS was 11.0 months, compared to 4.0 months for those with scores of 3-4 (P=0.18). The median OS was 41.8 months for ECOG 0-2 patients and 10.5 months for ECOG 3-4 patients (P=0.015).
Patients who had previously undergone four or more lines of therapy had significantly shorter PFS and OS compared to those who had received fewer than four lines (PFS: P=0.012; OS: P=0.0049). Among the 11 patients with EMD at enrollment, the ORR was 64% for those with EMD and 82% for those without EMD. However, median PFS and OS were notably shorter in patients with EMD (PFS: 3.0 months vs. 10.5 months, P=0.06; OS: 5.0 months vs. 24.0 months, P=0.03).
#### MRD-Negative Status and CAR-T Cell Persistence Indicate Better Long-Term Survival
Minimal residual disease (MRD) negativity was significantly associated with longer PFS and OS. In this study, MRD data were available for 22 patients on day 28 post-infusion, with 14 patients (63.64%) achieving MRD negativity (10^-4). These patients experienced significantly longer PFS and OS compared to MRD-positive patients. Similar associations were observed with MRD status at 3 and 6 months post-infusion.
The expansion of CAR-T cells was also closely related to clinical outcomes. Patients who achieved partial response (PR) or better had higher CAR-T cell peak levels. Patients without disease progression five years post-infusion had significantly higher CAR-T cell expansion peaks than those with progression. Additionally, the duration of CAR-T cell persistence correlated with longer PFS and OS, with patients having CAR-T cells persisting for ≥6 months, ≥12 months, ≥24 months, and ≥36 months showing significantly better PFS and OS than those without detectable CAR-T cells.
#### Controlled Safety Profile of BCMA CAR-T Cell Therapy HDS269B
No new CAR-T cell-related toxicities were observed during long-term follow-up. All patients experienced at least one adverse event (AE), with the most common long-term (≥28 days post-infusion) grade ≥3 AEs being hematologic in nature. No second primary malignancies or delayed immune effector cell-associated neurotoxicity syndrome (ICANS) were observed.
This study also included survival analysis, classifying patients by PFS and OS. The results indicated that ECOG 0-2 status, fewer than four prior therapies, and CAR-T cell persistence for ≥6 months were independently associated with longer survival.
### The Potential of BCMA CAR-T Therapy and the Need for Future Optimization
Through a five-year long-term follow-up of 49 RRMM patients, this study systematically evaluated the efficacy and safety of BCMA CAR-T cell therapy HDS269B. The findings suggest that poor physical status is not a contraindication for CAR-T therapy, thus broadening the indications for this treatment. While the results are encouraging, the study has some limitations, including its open-label, single-arm design and small sample size, which, combined with the long follow-up period, could lead to some patient attrition. Furthermore, despite the lack of new severe toxicities, long-term safety requires continued observation.
Overall, this study underscores the importance of BCMA CAR-T cell therapy in the treatment of RRMM and provides a crucial basis for exploring and applying CAR-T immunotherapy in the frontline treatment of multiple myeloma.

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +86137 1795 9070
Email: doctor.huang@globecancer.com
#CAR_Therapy #BCMACART #MultipleMyeloma #CancerResearch #LongTermSurvival #MedicalInnovation #Hematology #CancerTreatment #Immunotherapy #OncologyAdvances
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

#EBMT / #EHA Leading the Frontiers of Medicine: China’s Top Medical Expert Explains the Groundbreaking CAR-T Cell Therapy
#EBMT / #EHA Leading the Frontiers of Medicine: China’s Top Medical Expert Explains the Groundbreaking CAR-T Cell Therapy
Expert:**Huang He**
Chief Physician and Doctoral Supervisor.
Currently serves as the Director of the Hematology Institute at Zhejiang University, Director of the Zhejiang Provincial Engineering Research Center for Stem Cell and Cellular Immunotherapy, Chief Scientist for the “Hematology and Immunology Diseases” division at the Liangzhu Laboratory, and Director of the Bone Marrow Transplantation Center at the First Affiliated Hospital of Zhejiang University School of Medicine.
He is the Vice Chairman of the Asia Cell Therapy Organization and Deputy Leader of the Hematopoietic Stem Cell Application Group of the Hematology Branch of the Chinese Medical Association.

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in Chinafor preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in Chinafor preliminary evaluation!
WhatsApp:+8613717959070
Email: doctor.huang@globecancer.com
#MedicalInnovation #CAR_Therapy #Hematology #MedicalBreakthrough #ExpertTalks #CuttingEdgeMedicine #cart #EHA2024
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

The world’s first #CART therapy approved for second-line treatment of multiple myeloma is claimed by a Chinese pharmaceutical company
 The world’s first #CART therapy approved for second-line treatment of multiple myeloma is claimed by a Chinese pharmaceutical company
The world’s first #CART therapy approved for second-line treatment of multiple myeloma is claimed by a Chinese pharmaceutical company

Mutiple myeloma
#Legend Biotech
 How to advance #CARTcell therapy to frontline treatment is the goal every CAR-T company strives for. On April 5, 2024, local time, Chinese pharmaceutical company Legend Biotech (LEGN.NS) shared exciting news as the U.S. Food and Drug Administration (#FDA) officially approved #ciltacabtageneautoleucel for treating relapsed or refractory multiple myeloma patients. This significant decision brings new hope to patients who have undergone first-line treatment and developed resistance to standard therapies.
How to advance #CARTcell therapy to frontline treatment is the goal every CAR-T company strives for. On April 5, 2024, local time, Chinese pharmaceutical company Legend Biotech (LEGN.NS) shared exciting news as the U.S. Food and Drug Administration (#FDA) officially approved #ciltacabtageneautoleucel for treating relapsed or refractory multiple myeloma patients. This significant decision brings new hope to patients who have undergone first-line treatment and developed resistance to standard therapies.
#Ciltacabtagene autoleucel
 Ciltacabtagene autoleucel becomes the world’s first CAR-T product approved for second-line treatment! This milestone event paves the way for Legend Biotech to enter the global CAR-T market. What’s even more inspiring is that just the day before, the FDA approved BMS’s CAR-T therapy Abecma for third-line treatment of multiple myeloma. The approval of ciltacabtagene autoleucel signifies the strong competitiveness and innovation capabilities of Chinese pharmaceutical companies in the CAR-T field.
Ciltacabtagene autoleucel becomes the world’s first CAR-T product approved for second-line treatment! This milestone event paves the way for Legend Biotech to enter the global CAR-T market. What’s even more inspiring is that just the day before, the FDA approved BMS’s CAR-T therapy Abecma for third-line treatment of multiple myeloma. The approval of ciltacabtagene autoleucel signifies the strong competitiveness and innovation capabilities of Chinese pharmaceutical companies in the CAR-T field.
Marketing
 As early as February 28, 2022, ciltacabtagene autoleucel was first approved for marketing in the United States for the treatment of relapsed or refractory multiple myeloma in adults. As a pioneer of Chinese original CAR-T cell therapy, the successful market launch of this drug has won new glory for China’s biopharmaceutical industry. Currently, ciltacabtagene autoleucel is marketed in the United States, European Union, and Japan, and is also undergoing regulatory review in China, expected to be marketed domestically soon.
As early as February 28, 2022, ciltacabtagene autoleucel was first approved for marketing in the United States for the treatment of relapsed or refractory multiple myeloma in adults. As a pioneer of Chinese original CAR-T cell therapy, the successful market launch of this drug has won new glory for China’s biopharmaceutical industry. Currently, ciltacabtagene autoleucel is marketed in the United States, European Union, and Japan, and is also undergoing regulatory review in China, expected to be marketed domestically soon.
Personalized Immunotherapy
 The CEO of Legend Biotech stated that the expanded indication of ciltacabtagene autoleucel is expected to change the treatment landscape of multiple myeloma, providing physicians and patients with a personalized immunotherapy option for early treatment. Multiple myeloma is an incurable and progressive hematologic malignancy, thus urgently requiring innovative treatment options, for which ciltacabtagene autoleucel is designed.
The CEO of Legend Biotech stated that the expanded indication of ciltacabtagene autoleucel is expected to change the treatment landscape of multiple myeloma, providing physicians and patients with a personalized immunotherapy option for early treatment. Multiple myeloma is an incurable and progressive hematologic malignancy, thus urgently requiring innovative treatment options, for which ciltacabtagene autoleucel is designed.
HOPE
 The successful approval of ciltacabtagene autoleucel not only brings hope to patients but also outlines a new chapter for the future development of CAR-T therapy. In this hopeful moment, let’s look forward to CAR-T technology bringing health and happiness to more patients in the future!
The successful approval of ciltacabtagene autoleucel not only brings hope to patients but also outlines a new chapter for the future development of CAR-T therapy. In this hopeful moment, let’s look forward to CAR-T technology bringing health and happiness to more patients in the future!

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
Email: doctor.huang@globecancer.com,
WhatsApp: 137 1795 9070
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

🎉 #PNAS Breaking News: Revolutionary CAR-T Cell Therapy Successfully Treats Autoimmune Disease in China! 🎉
🎉 #PNAS Breaking News: Revolutionary CAR-T Cell Therapy Successfully Treats Autoimmune Disease in China! 🎉

PNAS
In a groundbreaking development, Chinese medical experts have achieved a milestone in the treatment of autoimmune diseases using CAR-T cell therapy.
🩺 A New Hope for Autoimmune Diseases
Autoimmune-mediated necrotizing myopathy (#IMNM) poses a significant challenge in clinical management due to its severe symptoms and limited efficacy of traditional pharmacological approaches. However, a ray of hope emerges with the application of Chimeric Antigen Receptor (CAR) T-cell therapy targeting B cell maturation antigen (#BCMA).
🔬 The Breakthrough Study
Published in the prestigious #journal PNAS, the study titled “Single-cell analysis of refractory anti-SRP necrotizing myopathy treated with anti-BCMA CAR-T cell therapy” by the medical team at Huazhong University of Science and Technology sheds light on a remarkable case. A patient with anti-signal recognition particle (SRP) IMNM exhibited significant improvement in clinical symptoms and sustained reduction in pathogenic autoantibodies after receiving BCMA-targeted CAR-T cell therapy for over 18 months.
🧬 Understanding the Mechanisms
Through longitudinal single-cell RNA sequencing and analysis of T cell receptors and B cell receptors, researchers elucidated the normalization of the immune microenvironment post-CAR-T cell infusion. The expansion of CD8+ CAR-T cells, coupled with dynamic phenotypic transitions in both CD4+ and CD8+ CAR-T cells, was observed, indicating a promising avenue for the treatment of autoimmune diseases.
🔍 Implications for the Future
This study not only highlights the efficacy of CAR-T cell therapy in refractory IMNM but also underscores the importance of understanding the molecular characteristics of CAR-T cells in autoimmune diseases. Further research is warranted to enhance the efficiency and durability of CAR-T cell therapy for autoimmune conditions.
🌟 Hope for Patients Worldwide
With this groundbreaking achievement, there is renewed hope for millions of individuals battling autoimmune diseases globally. Stay tuned for more updates as we continue to unlock the potential of innovative therapies in medical science!
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
Email: doctor.huang@globecancer.com,
WhatsApp: +8613717959070
#CAR_TTherapy #AutoimmuneDisease #MedicalBreakthrough #Science #Innovation #PNAS #cart #cartcell #BCMA #IMNM #chineseresearch #medicalscience
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

🍀Miraculous Journey Across Borders: Mr. Barankov’s Triumph with CAR-T Therapy in China 🍀
🍀Miraculous Journey Across Borders: Mr. Barankov’s Triumph with CAR-T Therapy in China 🍀

Hope of patients
🌱Meet Mr. Barankov
a 68-year-old patient from Saint Petersburg, Russia, who was unexpectedly diagnosed with multiple myeloma in 2018. After four years of international consultations, he discovered that China has made significant advancements in CAR-T therapy compared to European medical institutions in Spain, Munich, Heidelberg, and others.
🌿The treatment outcomes in China are leading globally.
Following unsuccessful traditional chemotherapy and two autologous hematopoietic stem cell transplants, he had the opportunity for an online consultation with experts from Jiahui International Hospital in Shanghai. During the virtual consultation, Mr. Barankov was impressed by the expertise of the Chinese medical team and the therapeutic potential of the fully human CAR-T drug FUCASO (Eque-cel). Considering China’s leading position in CAR-T therapy, more competitive costs, and relatively shorter wait times, Mr. Barankov made a significant decision to travel to China for treatment.
☘️#MiracleJourney #CARTTherapy #CrossingBorders #CART #Hopeofpatients #FUCASOApproval #Equecel #MultipleMyeloma #JiahuiHospital #Shanghai #Immunotherapy #MedicalInnovation #MedicalBreakthrough #CancerTreatment #FullyHumanCART
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese Medical Research!丨New Target CAR-T Cell Therapy in R/R MM Patients Sparks Hope and Shapes the Future
🌟Chinese Medical Research!🌟
丨New Target CAR-T Cell Therapy in R/R MM Patients Sparks Hope and Shapes the Future

GPRC5D, Multiple Myeloma
