Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

ความหวังใหม่สำหรับผู้ป่วยโรคมีเลือดมาก (Multiple Myeloma) – เริ่มต้นการรักษาด้วย CAR-T Therapy
🎯ความหวังใหม่สำหรับผู้ป่วยโรคมีเลือดมาก (Multiple Myeloma) – เริ่มต้นการรักษาด้วย CAR-T Therapy🎯
⭐เมื่อเร็ว ๆ นี้ ทีมของศาสตราจารย์ ดร. ลิ ปิง ณ โรงพยาบาลตงจิ ในเซี่ยงไฮ้ ได้ดำเนินการรักษาด้วยการภูมิคุ้มกันเซลล์ T ที่มีภูมิลักษณ์ผสม (Chimeric Antigen Receptor T-Cell Immunotherapy หรือ CAR-T) สำเร็จสำหรับผู้ป่วยโรคมีเลือดมากที่ซ้ำกลับและซ้ำแล้วและซ้ำไม่ได้ (R/RMM) นี่เป็นการรักษาที่เปิดโอกาสใหม่สำหรับการรักษาผู้ป่วย R/RMM
🌟ผู้ป่วยที่ได้รับการรักษาด้วย CAR-T นี้ได้รับการวินิจฉัยว่าเป็นโรคมีเลือดมากในเดือนกรกฎาคม พ.ศ. 2564 ในประเทศไทย โรคมีเลือดมากของผู้ป่วยมีภาระรักษามาก โรครุนแรง แม้จะได้รับการรักษาด้วยเคมีบำบัดระบบทั่วไป โมโนคลอนอนิบอดี้ การปลูกถ่ายเซลล์ลำไส้โภชนาการของตนเอง (ASCT) และการรักษาอื่น ๆ โรคยังกลับมาซ้ำกลับหลายครั้ง ในระยะเวลาของโรคเพียง 2 ปี เข้าสู่การรักษาหลายเส้นทาง และเจริญสามารถรับการรักษาอย่างเป็นตัวอย่าง ไม่สามารถควบคุมโรคได้อย่างมีประสิทธิภาพ
🌠 เมื่อจำนวนการเกิดการซ้ำกลับเพิ่มขึ้นในผู้ป่วย R/RMM และจำนวนของเส้นทางการรักษาขยายออก การตอบสนองต่อการรักษาก็เลวร้ายลงและระยะเวลาของการหายตัวยังกลับมาสั้นลง อย่างไรก็ตาม ด้วยการอนุมัติยา CAR-T therapy ที่เป็นมนุษย์ที่สมบูรณ์แบบแรกในโลก ชื่อ FUCASO (Eque-cel) ในจีนใหญ่ ความหวังใหม่ก็ได้ถูกเปิดขึ้นอย่างไม่สงบสำหรับการรักษาโรคมีเลือดมาก โครงสร้าง CAR ที่เป็นมนุษย์ที่สมบูรณ์แบบของมันไม่เพียงแต่มีความต้านทานต่อร่างกายน้อย แต่ยังมีการปลดตัวออกและการใช้งานต่ำ ทำให้ผู้ป่วยสามารถกลับไปสู่ระดับคุณภาพชีวิตที่สูงขึ้นด้วยการรักษาแค่ครั้งเดียว
☄หลังจากทีมของศาสตราจารย์ ดร. ลิ ปิง ประเมินพิจารณาพบว่าผู้ป่วยมีเงื่อนไขที่เหมาะสมสำหรับการรักษาด้วยเซลล์ CAR-T หลังจากการสื่อสารอย่างเต็มที่ระหว่างแพทย์กับผู้ป่วยและครอบครัวของเขา ผู้ป่วยในที่สุดก็เลือกที่จะรับการรักษาด้วย CAR-T และได้ทำการรวบรวมเซลล์โมโนเนวกลุ่มเลือดเป็นรายในเดือนพฤศจิกายน พ.ศ. 2566
✨ เราจะติดตามความคืบหน้าของการรักษาของผู้ป่วยต่อไปและรายงานติดตามต่อไป
#CARTTreatment #CARTTherapy #HopeReborn #FUCASOApproval #EquecelApproval #MultipleMyeloma #TongjiHospital #Shanghai #Immunotherapy #MedicalInnovation #MedicalBreakthrough #CancerTreatment #FullyHumanCART
หมายเหตุ: ภาพถ่ายและข้อมูลได้รับอนุญาตจากโรงพยาบาล ผู้ป่วย และครอบครัวของผู้ป่วย
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese CAR-T cell clinical and therapeutic have entered a period of rapid growth!
![]()
![]() **Chinese CAR-T cell clinical and therapeutic have entered a period of rapid growth!**
**Chinese CAR-T cell clinical and therapeutic have entered a period of rapid growth!** ![]()
![]()

Chinese CAR-T
According to the American clinical trial database Clinicaltrials, as of November 10, 2023, China has taken the lead in CAR-T cell therapy clinical research with a total of 655 studies, marking the country’s first foray into the international forefront of a new drug development field. Among them, Legend Biotech’s SicajioLunsei and Koji Pharmaceutical’s Claudin18.2 CAR-T have both achieved global leadership. Additionally, several companies are also advancing next-generation CAR-T and allogeneic CAR-T technologies, showing promising initial data and potential for global market success.

CAR-T therapy
“If you’d like to inquire about the latest cancer-fighting technologies and treatments, you can contact us.”
whatsapp: 137 1795 9070
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Breaking News: Another Milestone for Domestic CAR-T Therapy! Clinical Benefit Rate Reaches 71.4% in CT041 Trials, Challenging Gastric and Pancreatic Cancers with Astonishing Results!

Gastric Cancer, Pancreatic Cancer
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Miracle Unfolds: Zhejiang University Hospital’s Custom CRISPR/Cas9 Gene Editing + CAR-T Cell Therapy Rebirths Patients
🌟 Miracle Unfolds: Zhejiang University Hospital’s Custom CRISPR/Cas9 Gene Editing + CAR-T Cell Therapy Rebirths Patients 🌟
👨👧👦 (“I want to live to see my child go to university!”)👨👧👦

CAR-T Cell Therapy Patient
🔬14 rounds of chemotherapy, 20 rounds of radiotherapy, total gastrectomy… For two years,
🍋Seeking a glimmer of hope, he turned to Zhejiang University Hospital in Hangzhou. This time, the doctors said, “Let’s give it a try together.”
💪The brand-new PD1-19bbz CAR-T cells, with a low risk of cytokine storms and other complications, gave Mr. Sun a new lease on life.
🏸Today, Mr. Sun has been disease-free for over 2 years.

Tsinghua University
Email: doctor.huang@globecancer.com,
WhatsApp: +8613717959070
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Innovative Breakthrough: Chinese Team Creates Novel CAR-T Cells Using CRISPR/Cas9 Technology
🔬 Innovative Breakthrough: Chinese Team Creates Novel CAR-T Cells Using CRISPR/Cas9 Technology 🔬

Nature
💡 Analyzing the Scientific Principles behind the Technology 💡
🧬 Groundbreaking Clinical Validation 🧬
🌟 Pioneering a New Era in CAR-T Cell Therapy 🌟
💬 Expert Evaluation 💬
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

World’s First! Chinese Tongji Hospital Successfully Treats Immune-Mediated Necrotizing Myopathy with CAR-T Cells!
🎉🔬✨ World’s First! Chinese Tongji Hospital Successfully Treats Immune-Mediated Necrotizing Myopathy with CAR-T Cells! ✨🔬🎉

PNAS
🌟A groundbreaking achievement in medicine! Immune-Mediated Necrotizing Myopathy
Professor Wang Wei’s team from Tongji Hospital, affiliated with Huazhong University of Science and Technology, has brought unprecedented hope to patients with immune-mediated necrotizing myopathy. 🌟
📅 On January 30th, their research was published in the prestigious journal “Proceedings of the National Academy of Sciences” (PNAS). They utilized CAR-T cells targeting mature B cell antigen (BCMA) for the first time, achieving significant clinical efficacy in treating immune-mediated necrotizing myopathy. This research opens new avenues for treating this condition, marking a historic breakthrough in medicine.
ℹ️ CAR-T cell therapy
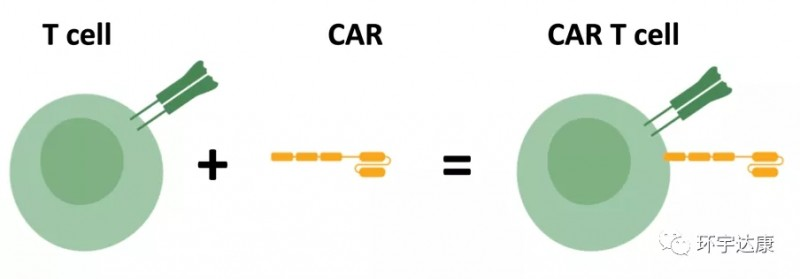
is a gene-editing cellular treatment method. Patient’s own T cells are extracted, genetically modified in vitro to possess targeted killing capabilities. These modified CAR-T cells are then reintroduced into the patient’s body to selectively eliminate target cells, bringing about dramatic changes in the disease’s progression.

Tongji
👨⚕️ Patient Mr. Deng is a successful case of CAR-T cell therapy. Diagnosed with immune-mediated necrotizing myopathy seven and a half years ago, his condition worsened over time, leaving him bedridden. After receiving CAR-T treatment, his health significantly improved, he successfully entered graduate school for medical studies, achieved full recovery, discontinued all immunosuppressive drugs, and now lives medication-free. This is a remarkable victory! 💪
🌈 What’s even more surprising is that several patients treated with CAR-T cells experienced the regrowth of new B cells approximately one year post-treatment. These new B cells exhibit a completely different phenotype, and pathogenic antibodies in the patients’ bodies completely disappear, achieving a joyful “immune reshaping.”
🌍 This breakthrough research brings hope to autoimmune disease patients worldwide.
Professor Wang Wei’s team’s efforts have paved the way for new treatment approaches, offering a fresh start for patients once deemed untreatable. We look forward to the global adoption of this treatment method, benefiting more patients worldwide. Let’s cheer for this remarkable achievement together! 👏
In addition to lung cancer, we are currently urgently recruiting patients with B-cell lymphoma, T-cell lymphoma, T-cell leukemia (T-ALL), acute lymphoblastic leukemia, myeloma, and other types of cancer!
You can send electronic copies or photos of genetic testing reports and diagnostic reports to the email address:
doctor.huang@globecancer.com,
or click on the
WhatsApp+8613717959070.
The Medical Department will contact you as soon as they receive the reports.🌟
#CARTCellTherapy #ImmuneMediated #NecrotizingMyopathy #MedicalBreakthrough #TongjiHospital #ScientificResearch #MedicalRevolution #HealthHope #myopathy
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

The Chinese CAR-T therapy achieves a miraculous cure for advanced liver cancer, creating wonders in the field of cellular treatment for solid tumors.
The Chinese CAR-T therapy achieves a miraculous cure for advanced liver cancer, creating wonders in the field of cellular treatment for solid tumors.🌞🌞

🥰Surviving Against the Odds: A Chinese Doctor’s Journey with Liver Tumor🥰
Zou began his career in 1989 and has dedicated 30 years as an obstetrician-gynecologist, tirelessly working on the frontline of clinical care.
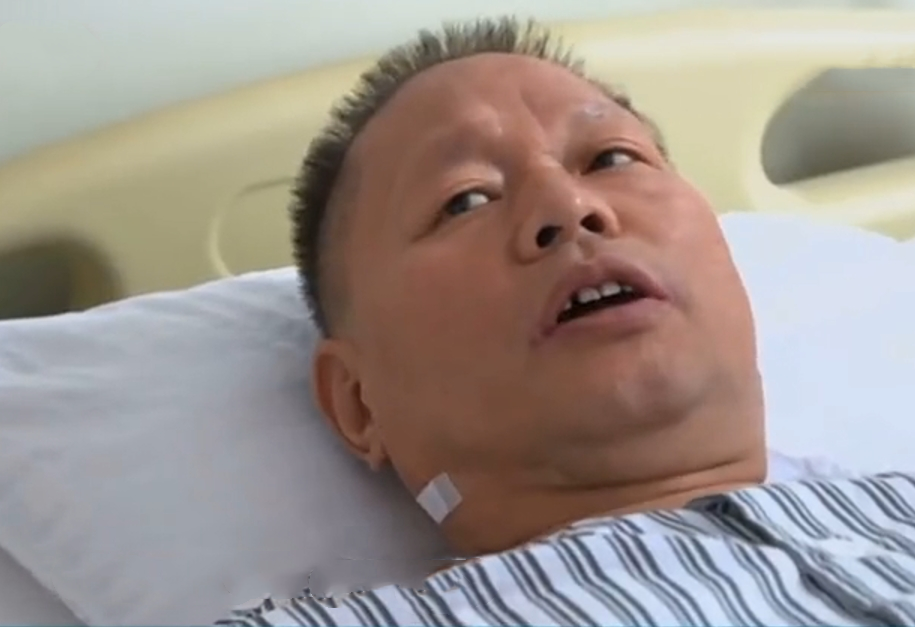
⭐️”Prof.Shi mentioned this immunotherapy, an antibody treatment,” said Dr. Zou’s wife.⭐️

Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

🥰Chinese CAR-T Therapy: A Beacon of Hope for Lymphoma Patients🥰
🥰Chinese CAR-T Therapy: A Beacon of Hope for Lymphoma Patients🥰
🌞In summary, CAR-T therapy presents a promising avenue for patients facing challenging lymphoma scenarios. The successful case of Mr. Li underscores the potential efficacy and safety of this innovative treatment at Peking Union Medical College Hospital.

Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
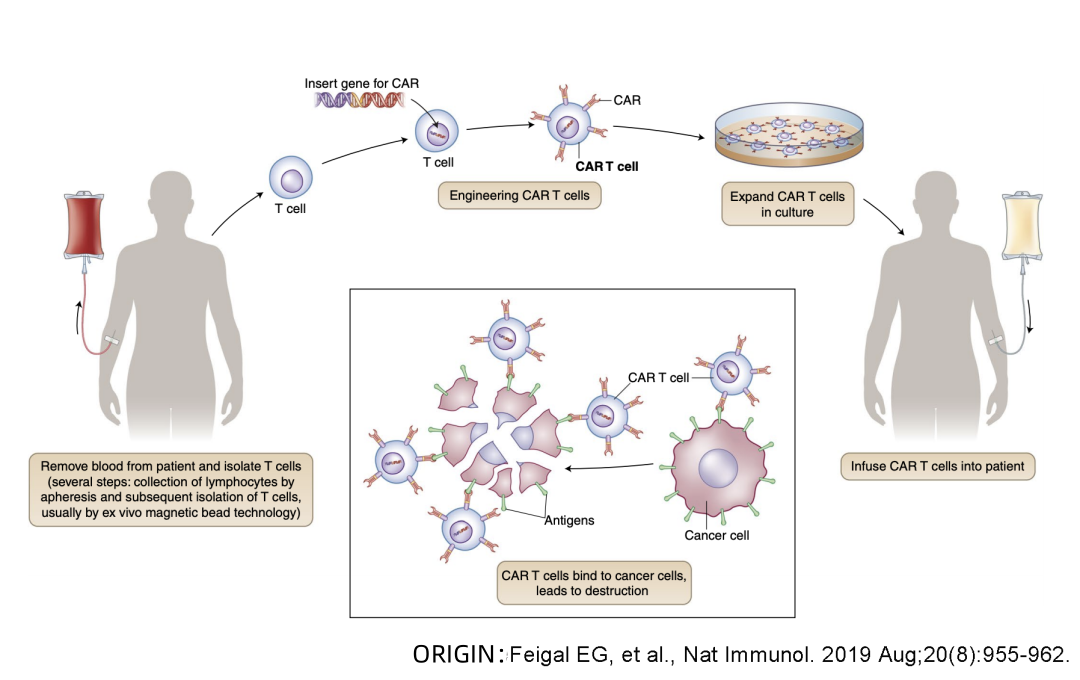
Beacon of Hope in Cancer Treatment – What is the treatment process for CAR-T cell therapy?
Beacon of Hope in Cancer Treatment
😊😊😊 What is the treatment process for CAR-T cell therapy?

Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

A Miraculous Journey: Israeli Artist Finds Cure for Multiple Myeloma in Hangzhou China






Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Breakthrough Ultra CAR-T Therapy Shows High Disease Control Rate of 85.7% in Advanced Platinum-Resistant Ovarian Cancer

Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Clinical Breakthrough: Chinese CAR-T – Inaticabtagene Autoleucel Revolutionizing Hematologic Cancer Therapy


