Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Why Does China Have a Unique Advantage in the Field of CAR-T Therapy?
**Why Does China Have a Unique Advantage in the Field of CAR-T Therapy?**

CAR-T
#CAR_Therapy #CancerTreatment #MedicalInnovation #ChinaMedical
In recent years, China has made rapid advancements in medical technology, particularly in the area of CAR-T (Chimeric Antigen Receptor T-cell) therapy. This cutting-edge cancer treatment involves reprogramming a patient’s own T cells to recognize and attack cancer cells. This revolutionary therapy has transformed treatment options for various malignancies, with China’s innovations and applications standing out globally.
### 1. **Scientific Innovation and Technological Breakthroughs**
China has reached a world-leading level in CAR-T research and development. Numerous prestigious medical schools and research institutions in China have actively contributed to this field, leading to the creation of several novel CAR-T treatment methods. Chinese scientists have pioneered significant innovations in CAR-T cell engineering, toxicity control, and target selection. For example, the CD19 CAR-T therapy for hematologic malignancies is widely used in China. Additionally, the fully human BCMA-targeted CAR-T product, Equecabtagene Autoleucel, used for multiple myeloma, has achieved a complete remission (CR) rate of 82.4%. Recently, *Nature* featured China’s breakthrough in using universal CAR-T for autoimmune diseases. New CAR-T therapies targeting solid tumors are also progressing rapidly.
### 2. **Cost-Effectiveness and Extensive Clinical Application**
Compared to some Western countries, CAR-T therapy in China is more affordable, partially due to lower production and R&D costs. A surge of local pharmaceutical and biotech companies has emerged, promoting the domestic production of CAR-T products and reducing reliance on imported drugs. This efficient supply chain management has not only lowered treatment costs but also made the personalized therapy more accessible to patients. While CAR-T therapy in the U.S. can cost $700,000–$800,000, the cost in China is about one-fifth to one-seventh of that.
Moreover, the faster approval process for clinical trials in China enables researchers to quickly bring new therapies to market, allowing patients earlier access to innovative treatments. This efficiency is supported by streamlined regulatory frameworks and healthcare reforms.
### 3. **Government Policy Support and International Collaboration**
The Chinese government has implemented various policies to strongly support biomedical innovation, including the development and application of CAR-T technology. For example, the National Medical Products Administration (NMPA) has accelerated the approval process for CAR-T therapies, simplifying regulatory procedures, and encouraging international cooperation and technology transfer. Many international pharmaceutical giants have partnered with Chinese companies to jointly advance CAR-T therapy research and commercialization. This openness has established China as a key global center for CAR-T treatment.
### 4. **Large Patient Population Driving Research and Application**
As the most populous country, China has a vast cancer patient population, providing abundant clinical cases and research resources for the application and further optimization of CAR-T therapy. This large patient base allows China to conduct large-scale clinical trials in a short time, accelerating the validation of treatment effectiveness and maturity. In China, not only are there local consensus guidelines for CAR-T usage, but each physician applying CAR-T has significant experience in both initial treatments and post-therapy management.
### 5. **Interdisciplinary Collaboration and Development**
China’s advancements in cellular immunotherapy are not solely dependent on progress in medical fields but are also supported by the integration of interdisciplinary technologies such as artificial intelligence, gene editing, and materials science. For instance, AI is increasingly playing a role in optimizing and personalizing CAR-T therapy designs. Gene editing tools like CRISPR are also widely applied in China for designing CAR-T cells, making them more precise and effective.
### Conclusion
China’s unique advantages in CAR-T therapy stem from continuous scientific breakthroughs, lower treatment costs, strong government support, and a vast patient base. As technology advances and international collaborations deepen, China is poised to play an increasingly important role in the global fight against cancer. CAR-T therapy offers new hope to cancer patients, and China’s innovation and strength are helping bring this hope to fruition sooner.
This is not just China’s advantage; it is a blessing for cancer patients worldwide.
🎉🎉To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#ChimericAntigenReceptor #CancerResearch #Immunotherapy #ChinaMedicalAdvancements #Biotech #GeneTherapy #CellTherapy #HealthcareRevolution #CancerCare #AIinHealthcare #PrecisionMedicine #GlobalHealth
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Nature Headline! China’s CAR-T Therapy Breakthrough Leads the World—A New Hope for Autoimmune Disease Treatment
🌟 **Nature Headline!** China’s CAR-T Therapy Breakthrough Leads the World—A New Hope for Autoimmune Disease Treatment

Autoimmune
#CAR_Therapy #CRISPR #AutoimmuneDisease #Immunotherapy #Nature #Autoimmune #IMNM #dcSSc #immunemediatednecrotizingmyopathy #cutaneoussystemicsclerosis
💪 Chinese medical scientists have made a major breakthrough in treating autoimmune diseases with revolutionary CAR-T therapy. This study, conducted by scientists from three Chinese universities, was published in the prestigious journal *Cell* on October 04, 2024. It is the first study to use universal CAR-T cells derived from healthy donors, successfully helping three autoimmune disease patients achieve long-term remission. This marks a new era in treating difficult-to-treat autoimmune diseases globally.
📈 Traditional CAR-T therapies are primarily used to treat cancer, relying on extracting immune cells from patients, modifying them genetically, and reinfusing them—an expensive and complex process. However, the Chinese research team developed a next-generation CAR-T therapy that uses gene-editing technology to modify donor-derived T cells. These cells can be mass-produced and are ready for immediate use. This new approach not only significantly reduces treatment costs but also shortens the preparation time.
In this study, three patients with severe autoimmune diseases received the universal CAR-T therapy, including a 57-year-old male patient, Mr. Gong, who suffered from systemic sclerosis. Just three days after treatment, his skin showed noticeable improvement, and he regained movement in his fingers. Two weeks later, he returned to work. Now, over a year of follow-up later, Mr. Gong’s condition remains stable, and he says he “feels great.” This demonstrates the therapy’s potential for long-term effectiveness and safety.
The development of this next-generation CAR-T therapy uses CRISPR-Cas9 gene-editing technology, allowing the modified cells to target and eliminate B cells—critical players in various autoimmune diseases. After treatment, the diseased B cells disappeared from patients, and healthy B cells regenerated, all without the occurrence of common severe side effects like cytokine release syndrome.
This achievement not only brings new hope for autoimmune disease treatment but also paves the way for future applications of CAR-T cell therapy. As clinical trials continue, more patients are expected to benefit from this groundbreaking treatment.
🎉🎉To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CAR_Therapy #GeneEditing #CRISPR #MedicalBreakthrough #AutoimmuneDisease #Immunotherapy #StemCellResearch #CellTherapy #Biotech #FutureOfMedicine #MedicalInnovation #HealthRevolution #ChineseScience #CancerResearch #MedicalTechnology #PrecisionMedicine #GlobalHealth #ScienceNews #HealthInnovation #CureForAll
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China’s New CAR-T Therapy ssCART-19: Exceptional Safety with Zero ICANS Incidence
China’s New CAR-T Therapy ssCART-19: Exceptional Safety with Zero ICANS Incidence

CARTTherapy
#ssCART19 #CAR_T #CART #ALL #ICANS #CNSL #CARTTherapy
CAR-T therapy has reached another breakthrough with the introduction of ssCART-19, a new treatment that leverages IL-6 gene silencing technology to significantly reduce side effects, particularly when treating relapsed/refractory acute lymphoblastic leukemia (r/r ALL). Unlike traditional CAR-T therapies, ssCART-19 drastically lowers the risks of severe cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS).
In the latest clinical trials, ssCART-19 demonstrated outstanding safety, with no patients experiencing ICANS, marking a milestone in CAR-T therapy safety. Among the 17 patients treated, 58.8% showed a significant increase in cell counts, and no cases of Grade 4 or higher CRS were reported. Additionally, ssCART-19 achieved an overall response rate (ORR) of 87.5% within three months, with nearly 63% of patients reaching complete remission.
The latest research also highlights that ssCART-19 outperforms traditional CAR-T products (cCART-19) in both safety and efficacy. Compared to conventional therapies, ssCART-19 significantly reduced the incidence of severe CRS, neutropenia, and ICANS, achieving an ORR of 91.5%. With these impressive results, ssCART-19 is expected to become a groundbreaking option for treating r/r ALL, offering new hope, especially for patients with central nervous system leukemia (CNSL).
Developed by a Chinese biopharmaceutical company, ssCART-19 has gained widespread recognition on the international stage, receiving Orphan Drug designation from the U.S. FDA and being listed as a breakthrough therapy in China. ssCART-19 offers a new treatment option for patients previously excluded from CAR-T therapy, greatly enhancing their chances of survival.
Stay tuned for more updates on how ssCART-19 is transforming cancer immunotherapy.

 To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CART #CancerTreatment #Leukemia #BreakthroughTherapy #InnovativeMedicine #Immunotherapy #CancerResearch
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Can the Million-dollar Anti-cancer Injection “Cure” Tumors?
Can the Million-dollar Anti-cancer Injection “Cure” Tumors?
Is CAR-T therapy really a “one-shot cure”?
And how can we ensure that this CAR “drives well and drives long”?
百万抗癌针能“治愈”肿瘤吗?
如何让CAR“开得好,开得久?”
Expert:
**Jing Pan**
**Associate Chief Physician**
**Department of Pediatric Hematology, Beijing Gaobo Hospital**
**Professional Memberships:**
– Member, Pediatric Oncology Professional Committee of the Chinese Anti-Cancer Association
– Youth Committee Member, Hematologic Oncology Professional Committee of the Chinese Anti-Cancer Association
– Youth Committee Member, Clinical Application Professional Committee of the Chinese Medical Biotechnology Association
**Expertise:**
Dr. Pan currently manages an 80-bed pediatric hematology unit and has extensive experience in pediatric hematology, particularly in CAR-T cell immunotherapy, with nearly 10 years of experience in the field. She is dedicated to stratified CAR-T treatment, optimizing the management of complications during CAR-T therapy, and establishing an efficacy monitoring system post-treatment. Dr. Pan and her team have accumulated one of the largest single-center case collections globally, particularly in the areas of sequential CAR-T therapy for improving long-term outcomes in B-ALL and in exploring autologous and allogeneic CD5, CD7 CAR-T therapy for T-ALL/LBL.
Her related clinical research on CD7 CAR-T, CD19 CAR-T, CD22 CAR-T, and CD19-22 sequential CAR-T has been published in leading international journals such as *Lancet Oncology*, *JCO*, *Blood*, *JHO*, and *Leukemia*. Additionally, she has frequently presented the latest advancements in her team’s immunotherapy research at both domestic and international conferences, including ASCO, ASH, EHA, and JSH.
潘 静
副主任医师
北京高博医院 小儿血液科
中国抗癌协会小儿肿瘤专业委员会专业委员
中国抗癌协会血液肿瘤专业委员会青年委员
中国医药生物技术协会医药生物技术临床应用专业委员会青年委员
擅长:
目前独立管理床位数80张的儿童血液病区。从事儿童血液科临床工作多年,特别是在儿童CAR-T细胞免疫治疗方面积累了近10年的经验。致力于CAR-T的分层治疗,优化CAR-T治疗过程中的并发症处理,建立CAR-T治疗后的疗效监控体系。尤其是其带领团队在序贯CAR-T提高B-ALL远期预后,T-ALL/LBL的自体、异体CD5、CD7CAR-T治疗探索方面目前积累了全球较大的单中心病例数。相关的CD7CAR-T临床研究、CD19CAR-T临床研究、CD22CAR-T临床研究、CD19-22序贯CAR-T研究等,发表在国际血液病权威杂志期刊Lancet on-cology、JC0、Blood、JHO和Leukemia。并多次在国内外学术会议(美国临床肿瘤大会ASCO、美国血液年会ASH、欧洲血液年会EHA、日本血液年会JSH)汇报团队免疫治疗的最新进展。

 To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

**Breakthrough in China’s CAR-T Therapy: 13-Year-Old Girl Overcomes Lupus, Opening New Hope for a Cure**
**Breakthrough in China’s CAR-T Therapy: 13-Year-Old Girl Overcomes Lupus, Opening New Hope for a Cure**

Lupus
#LupusTreatment #HopeForLupus #lupus #AutoimmuneDisease #ChinaHealthcare #SystemicLupusErythematosus #Erythematosus #patientstory #SLE
In June 2024, 13-year-old Qingqing experienced a major turning point in her life. A year after being diagnosed with systemic lupus erythematosus (SLE), she received innovative CAR-T therapy at Shanghai Children’s Medical Center and successfully achieved disease remission. SLE, often referred to as the “incurable cancer,” is an autoimmune disease that affects millions of people worldwide, severely damaging the brain, lungs, kidneys, and blood system. Even when not in an acute flare, patients often suffer from chronic symptoms such as fatigue, rashes, pain, and fever.
Qingqing’s treatment marks a significant breakthrough in the global fight against SLE. Previously, SLE patients could only rely on lifelong medications, such as steroids and immunosuppressants, to control the disease and prevent severe complications. However, these drugs often come with side effects and long-term organ damage. Qingqing’s case has offered the world new hope. A team led by Dr. Li Benshang, chief physician of the Hematology and Oncology Department, and Dr. Yin Lei, head of the Nephrology Department, pioneered the use of CAR-T therapy in SLE treatment and initiated a clinical study. After just two months of treatment, Qingqing’s condition has fully gone into remission, and all medications were discontinued.
CAR-T therapy was originally used in cancer treatment by extracting a patient’s T cells and genetically modifying them to recognize and kill abnormal cells in the body. In the treatment of SLE, CAR-T therapy eliminates the abnormal plasma cells producing autoantibodies, effectively “resetting” the patient’s immune system and addressing the root cause of the disease.
Qingqing’s success story is not only a milestone in China’s CAR-T technology but also a major leap forward in the global treatment of autoimmune diseases like SLE. Since the first CAR-T treatment cured an SLE patient in 2021, numerous clinical trials have been conducted worldwide, and several Chinese hospitals have also achieved success. However, while early results are encouraging, further research and validation are needed to confirm the long-term safety and efficacy of the treatment.
With 20% of the global population affected by various types of autoimmune diseases, CAR-T therapy holds the potential to bring new life to millions of patients. China’s ongoing research and international collaboration in this field offer unprecedented hope for overcoming persistent diseases like SLE.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
(Https://wa.me/+8613717959070)
Email: doctor.huang@globecancer.com
#CAR-Ttherapy #LupusTreatment #AutoimmuneDisease #MedicalInnovation #ChinaHealthcare #Immunotherapy #CancerTreatment #HealthBreakthrough #HopeForLupus #FutureOfMedicine
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

CAR-T Therapy in China: Relma-cel Offers Deep and Lasting Remission for Patients with Relapsed/Refractory Mantle Cell Lymphoma (MCL)
CAR-T Therapy in China: Relma-cel Offers Deep and Lasting Remission for Patients with Relapsed/Refractory Mantle Cell Lymphoma (MCL)

MCL
#MantleCellLymphoma #MCL #Lymphoma #RRMCL #RelmaCel #CART #Non-HodgkinLymphoma #NHL
In the global cancer treatment landscape, the advancements in CAR-T cell therapy are remarkable, particularly in the treatment of relapsed and refractory Mantle Cell Lymphoma (MCL). China’s CAR-T therapy, Relma-cel, has emerged as a new beacon of hope for patients, delivering deep and lasting remission for those who have undergone multiple lines of treatment.
Mantle Cell Lymphoma: A Complex and Challenging Disease
Mantle Cell Lymphoma (MCL) is a subtype of Non-Hodgkin Lymphoma (NHL) originating from B cells, accounting for 6% to 8% of all NHL cases. The disease typically affects older adults, and most patients are diagnosed at an advanced stage with poor prognostic factors. Despite the availability of various treatments, including Bruton’s Tyrosine Kinase inhibitors (BTKi), MCL remains difficult to cure, and patients often face recurrence or progression of the disease.
Relma-cel: A New Hope for Relapsed/Refractory MCL
In China, Relma-cel, a novel CAR-T cell therapy, was approved by the National Medical Products Administration (NMPA) in August 2024 for the treatment of adult patients with relapsed or refractory MCL who have failed multiple lines of therapy. This therapy offers a groundbreaking treatment option for MCL patients.
A Case Study: Deep Remission Achieved with CAR-T Therapy
A 51-year-old male MCL patient, after multiple treatment failures, eventually achieved complete remission through the CAR-T therapy Relma-cel. Diagnosed in 2014, the patient underwent R-CHOP chemotherapy, Rituximab-targeted therapy, as well as BTKi and PI3K inhibitor treatments. However, the disease continued to relapse with poor prognosis. Finally, in 2022, the patient achieved deep and lasting remission through CAR-T therapy. After CAR-T cell infusion, the patient reached complete remission within just one month and remained in remission for 22 months of follow-up.
Expert Commentary: The Future of CAR-T Therapy
Professor Li Ping from Tongji Hospital, Tongji University, highlighted that CAR-T cell therapy has broken through the limitations of traditional treatments for relapsed/refractory MCL, offering new hope for high-risk patients. For those who cannot benefit from conventional treatments, the introduction of CAR-T therapy is crucial, especially when applied early in the frontline treatment stage to maximize its efficacy.
Based on the latest clinical research data, Relma-cel has shown encouraging complete remission rates (67.8%) and objective response rates (81.36%) in treating relapsed/refractory MCL patients. These findings further underscore the importance of CAR-T cell therapy in patients who have failed BTKi treatment, positioning it as a potential first-choice treatment for these patients.
Globally, CAR-T therapy has become a standard treatment for relapsed/refractory MCL, particularly in third-line treatment settings. For patients with poor prognostic factors, CAR-T therapy may be key to overcoming the limitations of traditional treatments and extending survival.
Conclusion
As CAR-T cell therapy continues to evolve, more patients with relapsed/refractory MCL will have the opportunity to benefit from it. The clinical application of Relma-cel not only brings deep and lasting remission to patients but also sets a new example for the development of CAR-T therapy worldwide. With early intervention and precise treatment strategies, CAR-T therapy has the potential to create a brighter future for MCL patients.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
(Https://wa.me/+8613717959070)
Email: doctor.huang@globecancer.com
#CAR_T #CancerTreatment #RelmaCel #MCL #LymphomaAwareness #Oncology #Immunotherapy #MedicalInnovation #CancerResearch #ChinaHealthcare
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

**China’s CAR-T Therapy Approved for New Indication: Bringing New Hope to Relapsed Mantle Cell Lymphoma Patients**
**China’s CAR-T Therapy Approved for New Indication: Bringing New Hope to Relapsed Mantle Cell Lymphoma Patients**

MCL
The approval of Relmacabtagene Autoleucel brings renewed hope for the treatment of relapsed or refractory mantle cell lymphoma (R/R MCL). MCL is a rare and aggressive subtype of non-Hodgkin lymphoma (NHL). Despite advancements in treatment options over recent years, patients with R/R MCL continue to face high relapse rates and poor prognoses. Therefore, there is an urgent need for more effective and innovative therapies to improve treatment outcomes and survival expectations for these patients.
Relmacabtagene, a CD19-targeted autologous CAR-T cell therapy, works by precisely targeting and attacking cancerous B cells. It has shown remarkable efficacy in clinical studies. On August 27, 2024, the National Medical Products Administration (NMPA) officially approved the new indication for Relmacabtagene, allowing its use in adult patients with relapsed or refractory mantle cell lymphoma who have undergone at least two prior systemic therapies, including Bruton tyrosine kinase inhibitors (BTKi). This approval marks another significant milestone following Relmacabtagene’s previous approvals for R/R large B-cell lymphoma (LBCL) and follicular lymphoma (FL).
The treatment of R/R MCL has always been challenging. Traditional therapies often yield limited long-term results, particularly for patients who have failed BTKi treatment, leaving them with fewer options. The emergence of Relmacabtagene offers a new treatment avenue for these patients. Clinical studies demonstrate that this CAR-T cell therapy can significantly extend progression-free survival (PFS) and overall survival (OS), with a favorable safety profile.
The approval of this new indication not only expands the use of Relmacabtagene in treating R/R MCL but also paves the way for future advancements in MCL treatment. The application of CAR-T cell therapy, especially in high-risk patients, has shown potential in overcoming aggressive lymphomas. For patients with complex genetic characteristics and challenging treatment profiles, Relmacabtagene is undoubtedly a powerful therapeutic option.
Looking ahead, Relmacabtagene is expected to benefit more R/R MCL patients in clinical practice, pushing the boundaries of treatment in this field. As research deepens and new therapies continue to emerge, Relmacabtagene is poised to play an increasingly important role in improving patient outcomes and extending survival, offering new hope to R/R MCL patients worldwide.
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in Chinafor preliminary evaluation!
WhatsApp: +86137 1795 9070
(Http://wa.me/+8613717959070)
Email: doctor.huang@globecancer.com
#BloodCancerTreatment #CAR_TCellTherapy #CancerBreakthrough #Immunotherapy #BCMACART #MedicalAdvancements #CancerSurvivorship #ChinaMedicalInnovation #MCL #RRMCL #HopeForCancerPatients #Relmacabtagene #MCLTreatment #CancerBreakthrough #ChinaApproval #CAR_TCellTherapy #LymphomaTreatment #CancerInnovation #HematologyAdvances
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China’s Fully Human BCMA CAR-T Therapy: Bringing New Hope for High-Risk Relapsed/Refractory Multiple Myeloma
China’s Fully Human BCMA CAR-T Therapy: Bringing New Hope for High-Risk Relapsed/Refractory Multiple Myeloma

Mutiple Myeloma
Multiple Myeloma (MM) is a complex and aggressive cancer originating from plasma cells, posing significant treatment challenges due to its resistance to therapy. For patients with high-risk MM, especially those who experience relapse after multiple lines of treatment, finding an effective therapy is critical. Recently, Chinese medical team successfully treated a case of high-risk relapsed/refractory MM (RRMM) using fully human BCMA CAR-T cell therapy, offering new hope for such difficult cases.
**Case Overview**
The patient, a 56-year-old woman, presented with anemia during a routine check-up and was later diagnosed with MM. Despite undergoing several treatment regimens, including VRd (bortezomib, lenalidomide, dexamethasone) and Dara-DECP followed by autologous stem cell transplantation (ASCT), the disease continued to progress, with extramedullary disease (EMD) manifestations complicating the case.
**Challenges and Treatment Journey**
After initial treatments failed to achieve long-term remission, the patient’s condition worsened, with new lesions detected in the pancreas and multiple subcutaneous nodules indicating possible metastasis. Given the aggressive nature of the disease and the presence of EMD, which is associated with a poor prognosis, the medical team opted for a BCMA-targeted CAR-T cell therapy using Iquarense (Ikaros CAR-T), the first CAR-T product approved for MM treatment in China.
**CAR-T Therapy and Results**
Following pre-conditioning with a fludarabine-cyclophosphamide (FC) regimen, the patient received the BCMA CAR-T therapy. The treatment was well-tolerated, with only mild cytokine release syndrome (CRS) observed. Remarkably, within three months post-treatment, PET-CT scans showed no signs of active disease, and the patient achieved a complete response (CR), which has been sustained for eight months.
**Significance and Future Implications**
This case highlights the potential of BCMA CAR-T therapy as a powerful option for patients with high-risk, relapsed/refractory MM, particularly those with EMD. The successful outcome not only provides new hope for patients facing similar challenges but also contributes valuable insights for future treatment strategies. Iquarense, with its low immunogenicity and prolonged persistence in the body, represents a promising advance in the fight against this formidable disease.
For patients with high-risk MM, it is crucial to consider genetic factors, treatment response, and overall disease dynamics when selecting a therapeutic approach. As this case demonstrates, BCMA CAR-T therapy offers a viable path forward, particularly for those with limited options due to disease progression or EMD.
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
(Http://wa.me/+8613717959070)
Email: doctor.huang@globecancer.com
#BloodCancerTreatment #CAR_TCellTherapy #CancerBreakthrough #Immunotherapy #BCMACART #MedicalAdvancements #CancerSurvivorship #ChinaMedicalInnovation #HopeForCancerPatients
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
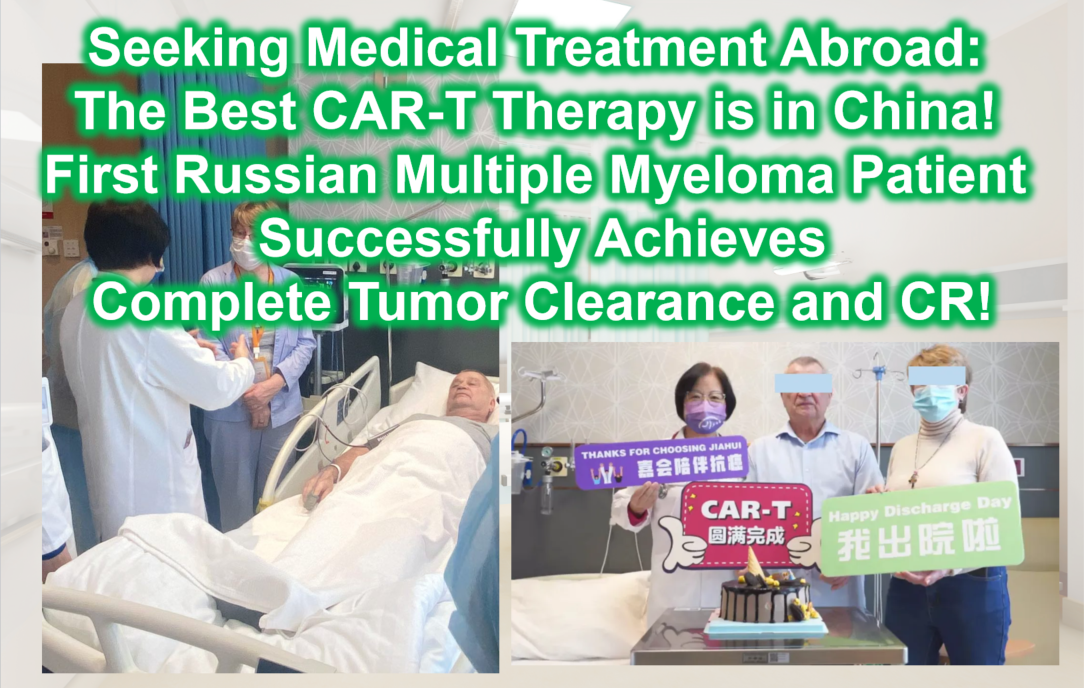
Seeking Medical Treatment Abroad: The Best CAR-T Therapy is in China! The First Russian Multiple Myeloma Patient Successfully Achieves Complete Tumor Clearance and CR!
**Seeking Medical Treatment Abroad: The Best CAR-T Therapy is in China! The First Russian Multiple Myeloma Patient Successfully Achieves Complete Tumor Clearance and CR!**

patient story
Over the five years following his diagnosis of high-risk multiple myeloma, Mr. F endured relentless pain. Despite undergoing various chemotherapy regimens and two transplants, he could never completely escape the nightmare of unpredictable tumor recurrence and progression. Fortunately, by the end of 2023, his condition temporarily stabilized. Accompanied by top hematology experts from Russia, Mr. F traveled to China to receive CAR-T therapy, achieving optimal results with complete tumor eradication and CR (complete remission).
Achieved CR! In February 2024, Mr. F and his family successfully returned to Russia to resume their happy lives.
(Note: To protect patient privacy, pseudonyms are used in the text, and the medical details and treatment process have been slightly modified.)

**Common Back Pain Turned Out to Be High-Risk Multiple Myeloma**
Mr. F, 63, is the vice principal of a technical college in St. Petersburg, Russia. He has a successful career and a happy family with his wife, one son, and two daughters. In the months leading up to Christmas 2017, Mr. F experienced worsening back pain, with severe pain in the lumbar region. Accompanied by his family, he went to the hospital for a thorough examination. The final diagnosis was multiple myeloma, with osteolytic lesions in more than three areas. The doctor informed Mr. F that, based on the comprehensive assessment of various test results, his myeloma was classified as high-risk (IgG-type, DS-Stage IIIA, ISS-Stage II), making it difficult to treat, prone to relapse, and with a poor prognosis. This devastating news struck suddenly, plunging the entire family into shock and panic. In an interview, Mr. F said, “In Chinese culture, Confucius said, ‘When Heaven is about to place a great responsibility on a man, it always first frustrates his spirit,’ viewing it as a life test. In Western culture, it is seen as God’s punishment, a trial one must endure, with the outcome entirely dependent on how one faces these challenges. I must choose to fight!”
**Relapse and Progression: The Unshakeable Nightmare for Multiple Myeloma Patients**
After two days of shock and panic, Mr. F decided to pull himself together and seek the best treatment to fight the tumor. Mr. F and his family consulted many doctors and clinics in Spain, Munich, and Heidelberg. Upon learning that CAR-T therapy had remarkable curative effects on hematologic malignancies, they felt a glimmer of hope, which was soon extinguished. At that time, very few countries offered CAR-T therapy, mostly in developed nations, and the exorbitant cost of nearly a million dollars made it unaffordable even for the well-off Mr. F. To control the disease as quickly as possible, Mr. F underwent traditional CVD therapy in Russia at the beginning of 2018, followed by his first autologous bone marrow transplant, achieving partial remission (PR). Unfortunately, just ten months later, his multiple myeloma relapsed. After switching to a second-line chemotherapy regimen, he underwent a second autologous bone marrow transplant. Despite receiving the most aggressive treatment and enduring life-threatening side effects, tumor lesions persisted in his body, like a ticking time bomb that could explode at any moment. Although maintenance therapy temporarily stabilized the disease, Mr. F and his family remained vigilant. They knew that high-risk myeloma was likely to relapse and progress again in the short term. If the disease worsened once more, they would face a desperate situation with no available treatments.

**Global Comparison Shows the Best CAR-T Therapy is in China!**
To completely rid himself of cancer and escape the nightmare of constant relapse and deterioration, five years later, Mr. F and his family once again sought the possibility of a cure through CAR-T therapy worldwide. They conducted extensive research on the CAR-T therapies already on the market and consulted numerous hospitals and experts in Europe, including in India and Israel. To his surprise, despite five years passing, there had been no significant progress in these countries. Almost every expert mentioned that Chinese hospitals had made tremendous advancements in the CAR-T field, surpassing even those in Europe and the United States. Especially for multiple myeloma, on June 30, 2023, China introduced the world’s first fully human BCMA CAR-T therapy, Iquilence. Mr. F and his attending physician immediately contacted Shanghai Jiahui International Hospital in China for an initial remote consultation. Jiahui Hospital, an internationally aligned oncology center with a professional multidisciplinary MDT team, conducted a preliminary evaluation. They concluded that the Iquilence (BCMA CAR-T) treatment plan would be most beneficial and developed a comprehensive plan, addressing all questions and concerns of Mr. F and the Russian experts, ultimately reaching a consensus on the treatment plan.
Professor Li Hua, Director of Oncology at Jiahui International Cancer Center, and his team of Russian hematology experts discussed Mr. F’s treatment plan. Although Mr. F was somewhat aware of China’s medical advancements, he couldn’t imagine that China’s healthcare had surpassed that of the developed Western countries. Therefore, in November 2023, Mr. F’s attending physician, a renowned Russian hematology expert, accompanied him to China to evaluate the feasibility of Chinese CAR-T therapy on-site. Their first stop upon arriving in China was to meet with Jiahui Hospital’s international multidisciplinary treatment team and tour the medical environment that adheres to international nursing standards.

Professor Li Hua, Director of Jiahui International Cancer Center, and his team provided a detailed introduction to the CAR-T treatment plan. Compared to traditional treatments, CAR-T has unique advantages. Firstly, it is a highly personalized treatment that adjusts the patient’s immune system through genetic engineering, providing a more precise response to cancer treatment. Secondly, CAR-T shows significantly higher efficacy in complex cases, which traditional treatments cannot achieve. Iquilence has an overall response rate of nearly 99% and a complete response rate of up to 82.4%. More importantly, fully human CAR-T not only maintains the patient’s quality of life but also provides more durable treatment effects. Additionally, a one-time infusion treatment achieves rapid and effective results, reducing the pain and burden during the treatment process and bringing hope for a cure.+

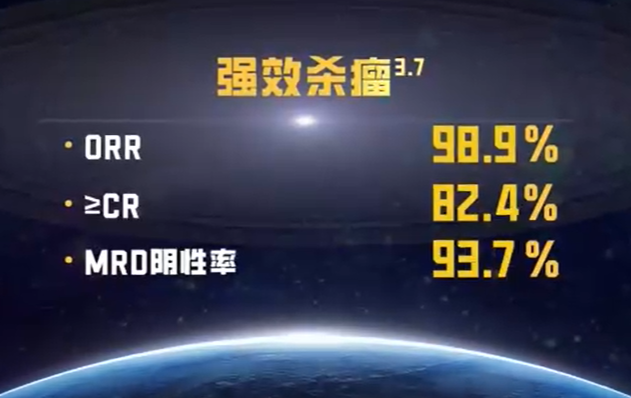
The second stop for Mr. F and the Russian experts was a visit to the production facility of the world’s first fully human BCMA CAR-T therapy, Iquilence. Mr. F and the Russian experts were impressed to learn that this exceptional therapy was not only entirely developed in-house, but the lentiviral vector was also independently developed. The facility boasts a complete end-to-end development platform and a self-built manufacturing base.

Finally, Mr. F overcame all his concerns and was convinced that China’s fully human BCMA CAR-T solution was world-leading. He decided to undergo treatment in China.
All Tumors Cleared for the First Time, Advanced Myeloma Achieves CR!
In December 2023, just before another Christmas five years after his diagnosis, Mr. F and his family arrived in China, full of hope for a cure, to officially begin his CAR-T journey. At Shanghai Jiahui International Hospital, Mr. F underwent thorough examinations, and after meeting all the requirements, he successfully underwent apheresis, the process of collecting T cells. These cells were then transported back to the factory within 24 hours to create a precisely targeted anti-cancer weapon tailored for Mr. F.
On January 20, 2024, he received lymphodepleting chemotherapy with fludarabine and cyclophosphamide.
On January 25, 2024, the CAR-T cells, carrying the hopes of Mr. F’s entire family, were transported to him by the pharmacy team using professional cold chain logistics. After multiple inspections, unpacking, and reactivation, a small bag of milky white liquid was infused back into Mr. F. The doctors explained that this liquid contained hundreds of billions of “special forces” T cells, which, once in the body, would begin an intense sweep to eradicate the myeloma cells completely.
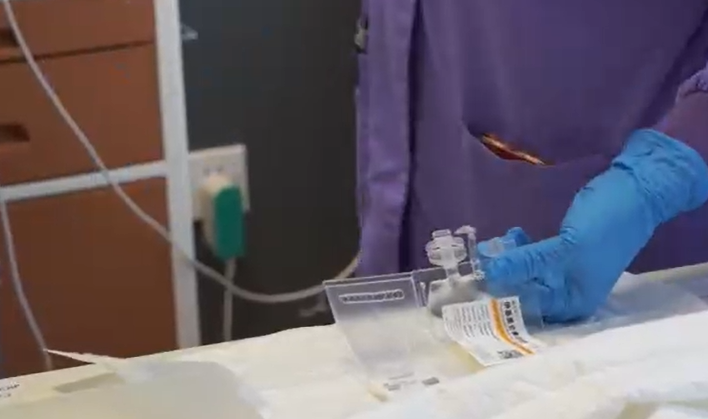
The infusion process went smoothly. What deeply moved Mr. F and his family was that whenever he felt anxious, the medical team would hold his hand, giving him confidence and strength.
Although a mild cytokine release syndrome (CRS), which is common after CAR-T therapy, occurred post-infusion, the medical team at Jiahui Hospital was very experienced in handling this. With symptomatic treatment, the symptoms improved in about a week. On the 14th day after the infusion, the evaluation showed that the CAR-T cells had expanded effectively in Mr. F’s body, reaching 10^9.
Mr. F said that, unlike the two painful transplant experiences, despite knowing that CAR-T therapy has side effects, the medical team clearly informed him about the reactions he might have at different stages and assured him that the medical staff would address them immediately. As a result, he felt no concerns or fears.
Before the Chinese New Year in 2024, Mr. F was discharged and returned to Russia for further follow-up in his home country. Professor Li Hua said, “The treatment results met our expectations, with significant improvements in key indicators such as serum M protein, free light chains, and disease symptoms. All functional statuses have improved.”
In May 2024, the PET-CT results showed that Mr. F’s lesions had disappeared, and he finally achieved complete remission (CR). This means that after battling multiple myeloma for six years, he has finally won!
A Brighter Future: China Will Become the Best Treatment Destination for Advanced Myeloma Patients!
Now, Mr. F not only resumes his happy and fulfilling life, but he and his wife have also returned to their beloved careers. He currently serves as the General Manager of the UNESCO Mineral Resources Education Center.
Mr. F said, “When I came to China and saw the medical conditions and experienced the best care firsthand, I felt a deep sense of pride for China. It is regrettable that Russia is currently unable to achieve this level of care. However, I will bring my experiences here back to Russia and share them. China’s medical system is world-leading, and choosing to come to China was the right decision!”

In an interview, Mr. F’s wife said, “Although we are past retirement age, we both have very important careers. This CAR-T treatment in China has given us a very precious experience. We have more time to listen to each other’s thoughts, support each other, and get through difficult times together.”

Professor Li Hua, Director of the International Oncology Hospital and Director of the Department of Oncology, said in an interview:
“Clinical research on CAR-T therapy started relatively early in China. Especially since 2020, the number of CAR-T clinical trials in China has far surpassed that in the United States, giving us extensive experience from laboratory research to clinical application.
Moreover, CAR-T treatment in China is more cost-effective, providing patients with a more competitive option.
Most importantly, China has sufficient commercial-quality production capacity. Patients can receive treatment quickly without waiting, which is crucial for those with malignant hematologic diseases who need timely treatment.”
Professor Li Hua, Director of Oncology at Jiahui International Hospital,
The emergence of CAR-T cell therapy has brought new hope to cancer patients worldwide. China’s CAR-T therapy offers outstanding efficacy, good safety, low cost, and rapid preparation advantages. Several international hospitals have opened green channels for overseas patients, making China the best treatment destination for global late-stage multiple myeloma patients!

References:
[1] Yuting Yan, et al. Blood Adv. 2019 Oct 8;3(19):2895-2904.[2] 2023 IMS. P-290.
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

22 Days from Desperation to Rebirth! Chinese CAR-T Therapy Creates Survival Miracle for Thai Multiple Myeloma Patient
22 Days from Desperation to Rebirth! Chinese CAR-T Therapy Creates Survival Miracle for Thai Multiple Myeloma Patient
In July 2017, 58-year-old Ms. M was diagnosed with multiple myeloma. After receiving treatments at the best hospital in Thailand, including PCD, DVD, bortezomib and lenalidomide, autologous hematopoietic stem cell transplantation. The disease rapidly relapsed. Local doctors informed Ms. M’s family that apart from palliative care, they were powerless.
Just as Ms. M was in dire straits, on June 30, 2023, the world’s first fully human BCMA CAR-T therapy – Equecabtagene Autoleucel, was shockingly launched in China, becoming a lifesaving straw for her. Ms. M and her family decided to seek treatment in China.
In September 2023, Professor Li Ping’s team at Shanghai Tongji Hospital developed a personalized Equecabtagene Autoleucel(BCMA CAR-T) therapy plan for Ms. M. What shocked everyone was that the examination results 22 days after CAR-T treatment showed that Ms. M had achieved hematologic CR, meaning that no cancer cells were detected in her blood. Her pain and anemia were also reversed. It was simply a miracle!
Reference:
[1]Yuting Yan, et al. Blood Adv. 2019 Oct 8;3(19):2895-2904.
[2] 2023 IMS. P-290.
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Nature Communications | Chinese Medical Team Innovates CAR-T Therapy to Bring New Hope for AML Treatment
Nature Communications | Chinese Medical Team Innovates CAR-T Therapy to Bring New Hope for AML Treatment

AML
In the field of hematological malignancies, the treatment of acute myeloid leukemia (AML) has long been a challenge in the medical community. Particularly for relapsed or refractory patients, the limitations of traditional treatments have increased the urgent need for new therapies. With the advancements in immunotherapy technology, the continuous updating of CAR-T cell therapy has brought new possibilities for the treatment of AML.
Recently, a study titled “CAR-T Cells with C-JUN Overexpression in Acute Myeloid Leukemia: Preclinical Features and Phase I Trial” was published in the journal Nature Communications. This study comprehensively explores the potential optimization mechanisms of CAR-T therapy in AML and was jointly completed by doctors and professors from three top hospitals in China.
During an interview, the Chinese medical team stated that in designing this study, they focused on the major obstacles in CAR-T cell therapy for AML. Although CD33, a highly expressed target, has been extensively explored in multiple studies, safety and efficacy remain challenges. The study found that high expression of CD155 affects the ERK signaling pathway, thereby hindering the effective expansion of CD33. To address this challenge, the research team ultimately selected the overexpression of C-JUN to enhance the exhaustion resistance of CAR-T cells by screening ERK pathway genes. Preliminary results from the clinical trial showed that this overexpressed CD33 CAR-T cell exhibited significant advantages in antitumor function.
Despite the progress, the challenges in CAR-T therapy for AML treatment were also emphasized, including the balance between high efficacy and low toxicity, the expansion of CAR-T cells in myeloid leukemia cells, and the affinity of antibody binding. Subsequent research will focus more on addressing these key issues to enable more patients to benefit from CAR-T therapy.
The Chinese clinical trials revealed some key findings of CAR-T therapy, such as the effectiveness of the bridging transplant strategy and the remarkable efficacy of CD33 CAR-T cells derived from transplant donors. These studies not only provide new insights into the application of CAR-T therapy in AML but also offer references for the optimization of therapies targeting other antigens.
The Chinese medical team will continue to conduct in-depth research around the three core issues of toxicity, expansion, and antigen binding, aiming to overcome challenges and enable more patients to benefit from the breakthrough results of CAR-T cell therapy.
����To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#CAR_TCellTherapy #AcuteMyeloidLeukemia #AMLResearch #ImmunotherapyAdvancements #ChineseMedicalInnovation #CancerTreatment #MedicalBreakthrough #NatureCommunications #OncologyResearch #HematologicalMalignancies
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Overcoming Challenges and Embracing New Life! – Breakthrough Progress of Eque-cel in the Treatment of Refractory Multiple Myeloma
### Overcoming Challenges and Embracing New Life! – Breakthrough Progress of Eque-cel in the Treatment of Refractory Multiple Myeloma

Multiple Myeloma
