Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

A significant breakthrough in Chinese solid tumor CAR-T therapy, resulting in complete remission for pancreatic cancer patients.

Global First: Chinese CAR-T Therapy Simultaneously Cures Tumor and Lupus Erythematosus

Unveiling Hope: Chinese CAR-T Cell Therapy Illuminating New Paths in Liver Cancer Treatment!
2020, over 19.3 million people were diagnosed with cancer worldwide, leading to almost 10 million fatalities. In China alone, the number of new cancer patients reached a staggering 4.57 million, accounting for 23.7% globally. Liver cancer, among the most prevalent malignant tumors in China, witnessed 410,000 new cases and 380,000 deaths, making up 45.3% and 47.1% of the global total, respectively [1]. However, since the 21st century began, significant strides have been made in liver cancer treatments, particularly in medication and localized therapies. Surgical procedures are no longer the sole option for long-term survival among liver cancer patients.
Immunotherapy has emerged as one of the most promising techniques for treating liver cancer, especially with advancements in tumor molecular biology. In 2013, “Science” magazine categorized immunotherapy as the fourth major cancer treatment, following surgery, chemotherapy, and radiation therapy, with cell therapy becoming a focal point of basic and clinical research in recent years.

In May 2020, Professor Zhai Bo’s team from Shanghai Jiao Tong University School of Medicine’s Renji Hospital, in collaboration with Shanghai Sci-Tech Biotechnology’s team led by Li Zonghai, published groundbreaking preliminary clinical research data on CAR-T cell therapy targeting the GPC3 gene for hepatocellular carcinoma in the “Clinical Cancer Research” journal. This breakthrough study brought unprecedented hope for CAR-T cell therapy in liver cancer treatment.

Even more inspiring, their publication in the “Cancer Communications” journal showcased follow-up results of two late-stage liver cancer patients who achieved long-term tumor-free survival after receiving CAR-T cell combined with local therapy [2]. These findings shed new light on the treatment prospects for liver cancer patients.
However, despite the potential therapeutic effects of liver cancer CAR-T cell therapy, it faces challenges and obstacles. Liver cancer’s heterogeneity, tumor microenvironment, and the safety of cell therapy remain crucial issues to address.
Presently, revolutionary changes are underway in the treatment models and concepts for liver cancer. However, integrating CAR-T cell therapy into actual liver cancer treatment requires further scientific exploration and clinical research. Researchers emphasize that only through comprehensive utilization of CAR-T cells in conjunction with other treatment modalities can its therapeutic potential be fully realized.
Professor Zhai Bo’s team is currently conducting various fundamental and clinical studies aimed at exploring additional possibilities for CAR-T cell therapy in solid tumors. These studies include phase I clinical research on EpCAM CAR-T cell combined with ablative therapy for gastrointestinal tumors, phase II clinical research on Claudin18.2 CAR-T cell therapy for gastrointestinal tumors, studies on the mechanism and prevention of OTOT toxicity, among others. These endeavors will provide more experimental data and support for the application of CAR-T cells in the treatment of solid tumors.
In conclusion, liver cancer CAR-T cell therapy signifies a significant breakthrough in the field of liver cancer treatment, offering new hope for patients. Despite the challenges to overcome, the outlook for this therapy is promising and holds the potential to bring a blessing to more patients in the future.
[References]
Zhaibo, Lizonghai etc.
“Chimeric Antigen Receptor-Glypican-3 T-Cell Therapy for Advanced Hepatocellular Carcinoma: Results of Phase 1 Trials.” 《Clinical Cancer Research》, 2020.
“Combined local therapy and CAR-GPC3 T-cell therapy in advanced hepatocellular carcinoma: a proof-of-concept treatment strategy.” 《Cancer Communications》, 2023.
#HealthTech#CancerResearch #Immunotherapy #CARTcell #LiverCancer #MedicalBreakthrough #ClinicalTrials #ScienceNews #HealthcareInnovation #ResearchBreakthrough #MedicalScience #CancerTherapy #CancerAwareness #InnovativeMedicine #ImmunotherapyTreatment #ScienceUpdates #HealthcareTechnology #BiomedicalResearch #ClinicalInnovation #CancerTreatment #MedicalAdvancements #ImmunologyResearch #HealthcareIndustry #ProfessionalHealthcare
The new hope for pediatric patients with relapsed/refractory B-cell acute lymphoblastic leukemia (R/R B-ALL) boasts an overall survival rate of up to 96%.


If a child unfortunately gets leukemia, should CAR-T Therapy be considered?

Breakthrough medical research in China brings hope for multiple myeloma patients.
Eque-cel brings new treatment prospects for multiple myeloma.
From December 9th to 12th, the Annual American Society of Hematology Meeting (ASH 2023) was held in San Diego, USA. At this conference, the FUMANBA-1 study, led by Professors Chunrui Li from Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, and Lugui Qiu from the Institute of Hematology, Chinese Academy of Medical Sciences, conducted at 14 medical centers nationwide, demonstrated the efficacy and characteristics of sustained minimal residual disease (MRD) negativity in multiple myeloma (MM) patients treated with Eque-cel injection (CT103A). Their achievements were included in the oral presentation at the conference.

Current MM Treatment and Challenges of CAR-T Therapy CAR-T cell therapy holds significant importance in the treatment of multiple myeloma, overcoming patients’ immunodeficiency and tolerance issues. Currently, China has approved three CAR-T cell drugs targeting multiple myeloma. Compared to traditional treatments, CAR-T cell therapy enhances immune function without relying on the major histocompatibility complex (MHC), improving the activity of NK cells, T cells, and B cells while effectively activating T cells. However, despite significant advancements in CAR-T cell therapy in the MM field, some patients still struggle to maintain treatment effectiveness, leading to potential relapses, with antigen escape being one of the important mechanisms causing relapse.
Furthermore, the safety of CAR-T therapy is a major concern, including adverse reactions such as cytokine release syndrome (CRS), neurotoxicity, infections, B cell depletion, and hypogammaglobulinemia. Hence, monitoring patient indicators throughout the entire CAR-T treatment process, from pre-treatment to infusion, is crucial for timely intervention in adverse reactions.
100% MRD Negativity Rate for CR and Above Patients! Eque-cel Injection Brings Redemption for Multiple Myeloma
Eque-cel injection (CT103A) is a fully humanized CAR-T cell therapy drug targeting B cell maturation antigen (BCMA), approved by the National Medical Products Administration (NMPA) for adult refractory/relapsed multiple myeloma patients in China. In the Phase II FUMANBA-1 study, this treatment showed significant and sustained efficacy.
As of December 31, 2022, with a median follow-up time of 18.07 months, deep and sustained remissions were observed in 103 evaluable patients. The overall response rate (ORR) among these 103 patients was 96.1%, with a strict complete response (sCR)/complete response (CR) rate of 77.7%. Among 91 subjects with no previous CAR-T treatment history, the ORR was 98.9%, and the sCR/CR rate reached 82.4%, with a 12-month progression-free survival (PFS) rate of 85.5%.
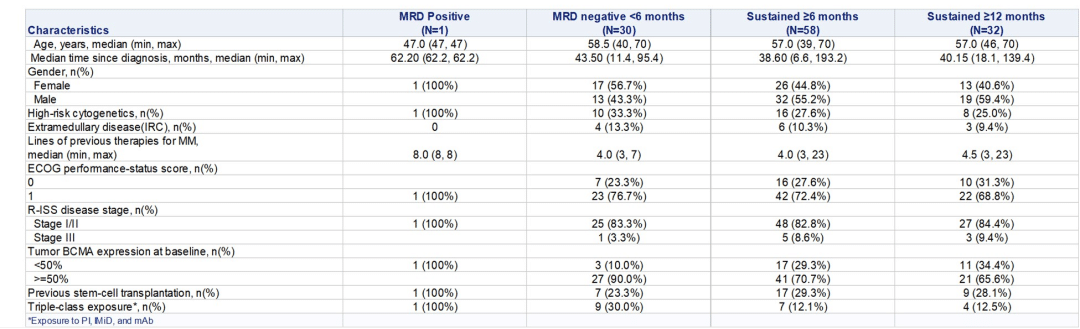
The minimal residual disease (MRD) negativity rate for the entire population was 94.2%, reaching 100% for CR and above patients. The median time to MRD negativity was 15 days, and 80.8% of patients maintained MRD negativity 12 months post-infusion. Moreover, the Eque-cel injection exhibited a relatively long median persistence in the body, lasting up to 307.5 days.
According to Professor Chunrui Li, “The sustained MRD-negative status is closely related to the improvement in PFS of patients receiving Eque-cel treatment.”
Professor Chunrui Li’s team studied the characteristics of patients maintaining MRD negativity for ≥6 months and ≥12 months in the FUMANBA-1 study. The research revealed that among 88 patients achieving MRD negativity, 78.4% maintained MRD negativity for at least 6 months, and 74.4% sustained MRD negativity for at least 12 months. Patients with sustained MRD negativity showed longer…

Professor Chunrui Li:
Chief Physician, Doctoral Supervisor
Secretary of the Hematology Department Party Committee, Deputy Director of Medicine Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology Professional
Focus: Immunotherapy for Hematological Malignancies
Member of the 11th Committee of the Plasma Cell Disease Professional Group of the Hematology Branch of the Chinese Medical Association
Chairman of the Expert Committee on Hematology (Hubei), Geriatrics Branch, Chinese Geriatrics and Gerontology Society
Standing Committee Member of the Geriatric Medicine Branch, Chinese Geriatrics and Gerontology Society
Youth Committee Member of the Oncology Branch, Chinese Anti-Cancer Association Committee Member of the Myeloma and Plasma Cell Disease Professional Group, 5th Committee of the Chinese Society of Clinical Oncology (CSCO) Leukemia Alliance & Lymphoma Alliance
Vice Chairman of the Blood Branch of the Hubei Medical Immunology Association Principal Investigator of four National Natural Science Foundation projects; published more than 20 SCI papers as first author or corresponding author, including Blood.
#EqueCelTreatment #MMResearch #CARTTherapy #MRDNegativity #CancerTreatment #MedicalBreakthrough #FUMANBA1Study #Immunotherapy #CancerResearchUpdate #CancerResearch #ASH2023 #BloodCancer #EqueCel #MRD #MultipleMyeloma
