Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Nature Headline! China’s CAR-T Therapy Breakthrough Leads the World—A New Hope for Autoimmune Disease Treatment
🌟 **Nature Headline!** China’s CAR-T Therapy Breakthrough Leads the World—A New Hope for Autoimmune Disease Treatment

Autoimmune
#CAR_Therapy #CRISPR #AutoimmuneDisease #Immunotherapy #Nature #Autoimmune #IMNM #dcSSc #immunemediatednecrotizingmyopathy #cutaneoussystemicsclerosis
💪 Chinese medical scientists have made a major breakthrough in treating autoimmune diseases with revolutionary CAR-T therapy. This study, conducted by scientists from three Chinese universities, was published in the prestigious journal *Cell* on October 04, 2024. It is the first study to use universal CAR-T cells derived from healthy donors, successfully helping three autoimmune disease patients achieve long-term remission. This marks a new era in treating difficult-to-treat autoimmune diseases globally.
📈 Traditional CAR-T therapies are primarily used to treat cancer, relying on extracting immune cells from patients, modifying them genetically, and reinfusing them—an expensive and complex process. However, the Chinese research team developed a next-generation CAR-T therapy that uses gene-editing technology to modify donor-derived T cells. These cells can be mass-produced and are ready for immediate use. This new approach not only significantly reduces treatment costs but also shortens the preparation time.
In this study, three patients with severe autoimmune diseases received the universal CAR-T therapy, including a 57-year-old male patient, Mr. Gong, who suffered from systemic sclerosis. Just three days after treatment, his skin showed noticeable improvement, and he regained movement in his fingers. Two weeks later, he returned to work. Now, over a year of follow-up later, Mr. Gong’s condition remains stable, and he says he “feels great.” This demonstrates the therapy’s potential for long-term effectiveness and safety.
The development of this next-generation CAR-T therapy uses CRISPR-Cas9 gene-editing technology, allowing the modified cells to target and eliminate B cells—critical players in various autoimmune diseases. After treatment, the diseased B cells disappeared from patients, and healthy B cells regenerated, all without the occurrence of common severe side effects like cytokine release syndrome.
This achievement not only brings new hope for autoimmune disease treatment but also paves the way for future applications of CAR-T cell therapy. As clinical trials continue, more patients are expected to benefit from this groundbreaking treatment.
🎉🎉To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CAR_Therapy #GeneEditing #CRISPR #MedicalBreakthrough #AutoimmuneDisease #Immunotherapy #StemCellResearch #CellTherapy #Biotech #FutureOfMedicine #MedicalInnovation #HealthRevolution #ChineseScience #CancerResearch #MedicalTechnology #PrecisionMedicine #GlobalHealth #ScienceNews #HealthInnovation #CureForAll
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

The significance of the 11 CD19 and BCMA CAR-T therapies currently approvedin China and the U.S. forpatients with hematologic malignancies.
The significance of the 11 CD19 and BCMA CAR-T therapies currently approvedin China and the U.S. forpatients with hematologic malignancies.
目前中美上市的11款CD19和BCMA CAR-T对血液瘤患者的意义
Expert:
**Jing Pan**
**Associate Chief Physician**
**Department of Pediatric Hematology, Beijing Gaobo Hospital**
**Professional Memberships:**
– Member, Pediatric Oncology Professional Committee of the Chinese Anti-Cancer Association
– Youth Committee Member, Hematologic Oncology Professional Committee of the Chinese Anti-Cancer Association
– Youth Committee Member, Clinical Application Professional Committee of the Chinese Medical Biotechnology Association
**Expertise:**
Dr. Pan currently manages an 80-bed pediatric hematology unit and has extensive experience in pediatric hematology, particularly in CAR-T cell immunotherapy, with nearly 10 years of experience in the field. She is dedicated to stratified CAR-T treatment, optimizing the management of complications during CAR-T therapy, and establishing an efficacy monitoring system post-treatment. Dr. Pan and her team have accumulated one of the largest single-center case collections globally, particularly in the areas of sequential CAR-T therapy for improving long-term outcomes in B-ALL and in exploring autologous and allogeneic CD5, CD7 CAR-T therapy for T-ALL/LBL.
Her related clinical research on CD7 CAR-T, CD19 CAR-T, CD22 CAR-T, and CD19-22 sequential CAR-T has been published in leading international journals such as *Lancet Oncology*, *JCO*, *Blood*, *JHO*, and *Leukemia*. Additionally, she has frequently presented the latest advancements in her team’s immunotherapy research at both domestic and international conferences, including ASCO, ASH, EHA, and JSH.
潘 静
副主任医师
北京高博医院 小儿血液科
中国抗癌协会小儿肿瘤专业委员会专业委员
中国抗癌协会血液肿瘤专业委员会青年委员
中国医药生物技术协会医药生物技术临床应用专业委员会青年委员
擅长:
目前独立管理床位数80张的儿童血液病区。从事儿童血液科临床工作多年,特别是在儿童CAR-T细胞免疫治疗方面积累了近10年的经验。致力于CAR-T的分层治疗,优化CAR-T治疗过程中的并发症处理,建立CAR-T治疗后的疗效监控体系。尤其是其带领团队在序贯CAR-T提高B-ALL远期预后,T-ALL/LBL的自体、异体CD5、CD7CAR-T治疗探索方面目前积累了全球较大的单中心病例数。相关的CD7CAR-T临床研究、CD19CAR-T临床研究、CD22CAR-T临床研究、CD19-22序贯CAR-T研究等,发表在国际血液病权威杂志期刊Lancet on-cology、JC0、Blood、JHO和Leukemia。并多次在国内外学术会议(美国临床肿瘤大会ASCO、美国血液年会ASH、欧洲血液年会EHA、日本血液年会JSH)汇报团队免疫治疗的最新进展。

 To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#cart #carttherapy #CAR_T #leukemia #lymphoma #cancer #tumor #bloodcancer
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Why More and More Foreigners Are Choosing China for CAR-T Cell Therapy
**Why More and More Foreigners Are Choosing China for CAR-T Cell Therapy**

CAR-T
In recent years, China’s advancements in cell therapy, particularly CAR-T therapy, have been remarkable, attracting numerous international patients seeking treatment. With its cutting-edge technology, reasonable costs, and outstanding efficacy, China has become a preferred destination for cancer patients worldwide. Today, we will explore why an increasing number of foreign patients choose to come to China for cell therapy.
**The Rise of CAR-T Therapy: China’s Advantages**
First, CAR-T therapy is a personalized immunotherapy that specifically targets blood-related cancers such as lymphoma, multiple myeloma, and leukemia. By engineering a patient’s own T-cells to recognize and attack cancer cells, CAR-T therapy has shown significant efficacy, especially in patients with advanced cancer.
China’s advantages in cell therapy can be summarized into three key points:
-
**Technological Expertise**: China has accumulated extensive experience in CAR-T therapy, particularly in top hospitals in major cities like Shanghai and Beijing. These institutions boast world-class medical teams and advanced treatment systems, providing international patients with high-quality treatment plans that ensure global leading outcomes.
-
**Affordable Costs**: While CAR-T therapy can cost millions of dollars in countries like the U.S. and Europe, the treatment in China is much more affordable, usually about one-seventh to one-tenth of the cost in the U.S. This affordability makes China an attractive option for many international patients.
-
**Shorter Waiting Times**: In Western countries, patients often wait several months for CAR-T therapy, whereas in China, many hospitals can complete the entire process — from diagnosis to cell infusion — in just a few weeks. This is crucial for patients with urgent medical needs.
**Real-Life Cases: Why Foreign Patients Choose China**
Several real-life cases clearly demonstrate why international patients are increasingly seeking treatment in China.
– **Ethan from Singapore**
Ethan had been battling cancer for nearly ten years, trying various treatments globally with little success. Eventually, he decided to seek treatment in China. After a thorough evaluation at Jiahui International Hospital in Shanghai, Ethan received a personalized CAR-T therapy plan. Within just four weeks, his cancer cells had significantly decreased, and his health began to improve, along with his weight. Ethan remarked, “The treatment in China is not only effective but also much more affordable than in Singapore.”
– **A Russian Patient’s Story**
Borzinkov, a 70-year-old patient with high-risk advanced multiple myeloma, had experienced multiple treatment failures before learning about China’s CAR-T therapy. He traveled to China for treatment, successfully completed cell collection, and received CAR-T infusion. Just 13 days later, he was discharged, and his follow-up results have been very promising. He chose China not only for the lower costs but also because of the shorter waiting time.
These cases illustrate that international patients in China can receive high-quality, effective treatment at more affordable rates and with faster recovery times. So far, patients from countries like Russia, Singapore, Malaysia, and others have undergone CAR-T therapy in China and achieved significant results, with many experiencing complete remission within months, with no signs of cancer after treatment.
**China’s Leading Position and Future Prospects**
China has already established itself as a global leader in CAR-T therapy, accounting for over 50% of the world’s clinical trials in this field. Chinese researchers and doctors excel not only in the basic research and clinical applications of CAR-T therapy but also in managing side effects such as CRS and neurotoxicity.
In cities like Shanghai, Wuhan, and Beijing, doctors provide cutting-edge treatment to international patients daily, consistently delivering excellent outcomes. As China’s medical system continues to advance, more and more international patients are choosing China as their destination for treatment.
**Conclusion**
The reasons why foreign patients are choosing China for cell therapy are clear: China excels in technology, offers reasonable treatment costs, and delivers outstanding results. As the world becomes more aware of the progress in China’s healthcare system, more patients will seek advanced medical care in China. In particular, China’s breakthroughs in cell therapy will continue to attract cancer patients from across the globe.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can contact us for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CARtherapy #CellTherapy #CancerTreatment #Immunotherapy #HealthcareInnovation #MedicalTourism #ChinaHealthcare #CancerSurvivor #PatientStories #MedicalAdvancements #AffordableHealthcare #PersonalizedMedicine #GlobalHealth #HealthJourney #Oncology #CAR-T
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

The Courage to Be First: China’s CAR-T Pioneer, “Emily of China,” Celebrates 6 Years in Complete Remission!
### The Courage to Be First: China’s CAR-T Pioneer, “Emily of China,” Celebrates 6 Years in Complete Remission!

Patient Story
#CART #PatientStory #lymphoma #TP53 #DLBCL #CARTTherapy
Meet Mrs. Qin, a true fighter and the first brave patient in China to receive Fosun Kite’s Axicabtagene Ciloleucel in a CAR-T clinical trial. Her story is one of hope, perseverance, and the power of innovation.
In early 2017, a routine check-up uncovered a small lump in her neck, leading to a diagnosis of diffuse large B-cell lymphoma (DLBCL) with a TP53 gene mutation—a high-risk condition. After enduring 12 rounds of grueling chemotherapy, the cancer still returned. Just when hope seemed to be fading, Mrs. Qin became the first patient in China to join a groundbreaking CAR-T therapy trial at Ruijin Hospital under the care of Dr. Zhao Weili. She was a pioneer, much like Emily Whitehead in the U.S., the world’s first pediatric patient to receive CAR-T therapy.
Mrs. Qin recalls the challenging days of her treatment, which involved high fevers and fatigue, yet the unwavering support of Dr. Zhao’s team helped her push through. The cutting-edge CAR-T treatment not only controlled the tumor but led her into complete remission. Six years later, she remains cancer-free and now serves as a volunteer at the hospital, inspiring others who are fighting the same battle.
During the first “CAR-T Therapy Survivor Conference” in Wuhan of China, Mrs. Qin took the stage to share her emotional journey. Her story moved everyone in attendance, from fellow patients to medical experts, as she expressed gratitude for the love and care she received. “Ruijin Hospital is not just where I found a new lease on life, but a home filled with compassion and world-class medical expertise,” she shared.
Mrs. Qin’s tale of courage is a beacon of hope for those still battling the disease. Her message is simple: “No matter how tough the road, never lose faith. After every storm, the rainbow will appear.”
🎉🎉To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#ChinaCAR-T #CAR-TTherapy #CancerSurvivor #MedicalBreakthrough #FosunKite #DLBCL #CancerRemission #InnovativeMedicine #HopeHealing #RuijinHospital #ChineseMedicalPioneers #PatientStories #EmilyofChina
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Can the Million-dollar Anti-cancer Injection “Cure” Tumors?
Can the Million-dollar Anti-cancer Injection “Cure” Tumors?
Is CAR-T therapy really a “one-shot cure”?
And how can we ensure that this CAR “drives well and drives long”?
百万抗癌针能“治愈”肿瘤吗?
如何让CAR“开得好,开得久?”
Expert:
**Jing Pan**
**Associate Chief Physician**
**Department of Pediatric Hematology, Beijing Gaobo Hospital**
**Professional Memberships:**
– Member, Pediatric Oncology Professional Committee of the Chinese Anti-Cancer Association
– Youth Committee Member, Hematologic Oncology Professional Committee of the Chinese Anti-Cancer Association
– Youth Committee Member, Clinical Application Professional Committee of the Chinese Medical Biotechnology Association
**Expertise:**
Dr. Pan currently manages an 80-bed pediatric hematology unit and has extensive experience in pediatric hematology, particularly in CAR-T cell immunotherapy, with nearly 10 years of experience in the field. She is dedicated to stratified CAR-T treatment, optimizing the management of complications during CAR-T therapy, and establishing an efficacy monitoring system post-treatment. Dr. Pan and her team have accumulated one of the largest single-center case collections globally, particularly in the areas of sequential CAR-T therapy for improving long-term outcomes in B-ALL and in exploring autologous and allogeneic CD5, CD7 CAR-T therapy for T-ALL/LBL.
Her related clinical research on CD7 CAR-T, CD19 CAR-T, CD22 CAR-T, and CD19-22 sequential CAR-T has been published in leading international journals such as *Lancet Oncology*, *JCO*, *Blood*, *JHO*, and *Leukemia*. Additionally, she has frequently presented the latest advancements in her team’s immunotherapy research at both domestic and international conferences, including ASCO, ASH, EHA, and JSH.
潘 静
副主任医师
北京高博医院 小儿血液科
中国抗癌协会小儿肿瘤专业委员会专业委员
中国抗癌协会血液肿瘤专业委员会青年委员
中国医药生物技术协会医药生物技术临床应用专业委员会青年委员
擅长:
目前独立管理床位数80张的儿童血液病区。从事儿童血液科临床工作多年,特别是在儿童CAR-T细胞免疫治疗方面积累了近10年的经验。致力于CAR-T的分层治疗,优化CAR-T治疗过程中的并发症处理,建立CAR-T治疗后的疗效监控体系。尤其是其带领团队在序贯CAR-T提高B-ALL远期预后,T-ALL/LBL的自体、异体CD5、CD7CAR-T治疗探索方面目前积累了全球较大的单中心病例数。相关的CD7CAR-T临床研究、CD19CAR-T临床研究、CD22CAR-T临床研究、CD19-22序贯CAR-T研究等,发表在国际血液病权威杂志期刊Lancet on-cology、JC0、Blood、JHO和Leukemia。并多次在国内外学术会议(美国临床肿瘤大会ASCO、美国血液年会ASH、欧洲血液年会EHA、日本血液年会JSH)汇报团队免疫治疗的最新进展。

 To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

**Breakthrough in China’s CAR-T Therapy: 13-Year-Old Girl Overcomes Lupus, Opening New Hope for a Cure**
**Breakthrough in China’s CAR-T Therapy: 13-Year-Old Girl Overcomes Lupus, Opening New Hope for a Cure**

Lupus
#LupusTreatment #HopeForLupus #lupus #AutoimmuneDisease #ChinaHealthcare #SystemicLupusErythematosus #Erythematosus #patientstory #SLE
In June 2024, 13-year-old Qingqing experienced a major turning point in her life. A year after being diagnosed with systemic lupus erythematosus (SLE), she received innovative CAR-T therapy at Shanghai Children’s Medical Center and successfully achieved disease remission. SLE, often referred to as the “incurable cancer,” is an autoimmune disease that affects millions of people worldwide, severely damaging the brain, lungs, kidneys, and blood system. Even when not in an acute flare, patients often suffer from chronic symptoms such as fatigue, rashes, pain, and fever.
Qingqing’s treatment marks a significant breakthrough in the global fight against SLE. Previously, SLE patients could only rely on lifelong medications, such as steroids and immunosuppressants, to control the disease and prevent severe complications. However, these drugs often come with side effects and long-term organ damage. Qingqing’s case has offered the world new hope. A team led by Dr. Li Benshang, chief physician of the Hematology and Oncology Department, and Dr. Yin Lei, head of the Nephrology Department, pioneered the use of CAR-T therapy in SLE treatment and initiated a clinical study. After just two months of treatment, Qingqing’s condition has fully gone into remission, and all medications were discontinued.
CAR-T therapy was originally used in cancer treatment by extracting a patient’s T cells and genetically modifying them to recognize and kill abnormal cells in the body. In the treatment of SLE, CAR-T therapy eliminates the abnormal plasma cells producing autoantibodies, effectively “resetting” the patient’s immune system and addressing the root cause of the disease.
Qingqing’s success story is not only a milestone in China’s CAR-T technology but also a major leap forward in the global treatment of autoimmune diseases like SLE. Since the first CAR-T treatment cured an SLE patient in 2021, numerous clinical trials have been conducted worldwide, and several Chinese hospitals have also achieved success. However, while early results are encouraging, further research and validation are needed to confirm the long-term safety and efficacy of the treatment.
With 20% of the global population affected by various types of autoimmune diseases, CAR-T therapy holds the potential to bring new life to millions of patients. China’s ongoing research and international collaboration in this field offer unprecedented hope for overcoming persistent diseases like SLE.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
(Https://wa.me/+8613717959070)
Email: doctor.huang@globecancer.com
#CAR-Ttherapy #LupusTreatment #AutoimmuneDisease #MedicalInnovation #ChinaHealthcare #Immunotherapy #CancerTreatment #HealthBreakthrough #HopeForLupus #FutureOfMedicine
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China’s first BCMA CAR-T therapy successfully treats an overseas patient with advanced relapsed multiple myeloma
China’s first BCMA CAR-T therapy successfully treats an overseas patient with advanced relapsed myeloma.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp:+8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#MultipleMyeloma #CAR-T #CancerTreatment #MedicalBreakthrough #Oncology #HealthcareInnovation #BCMACART
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

**China’s CAR-T Therapy Approved for New Indication: Bringing New Hope to Relapsed Mantle Cell Lymphoma Patients**
**China’s CAR-T Therapy Approved for New Indication: Bringing New Hope to Relapsed Mantle Cell Lymphoma Patients**

MCL
The approval of Relmacabtagene Autoleucel brings renewed hope for the treatment of relapsed or refractory mantle cell lymphoma (R/R MCL). MCL is a rare and aggressive subtype of non-Hodgkin lymphoma (NHL). Despite advancements in treatment options over recent years, patients with R/R MCL continue to face high relapse rates and poor prognoses. Therefore, there is an urgent need for more effective and innovative therapies to improve treatment outcomes and survival expectations for these patients.
Relmacabtagene, a CD19-targeted autologous CAR-T cell therapy, works by precisely targeting and attacking cancerous B cells. It has shown remarkable efficacy in clinical studies. On August 27, 2024, the National Medical Products Administration (NMPA) officially approved the new indication for Relmacabtagene, allowing its use in adult patients with relapsed or refractory mantle cell lymphoma who have undergone at least two prior systemic therapies, including Bruton tyrosine kinase inhibitors (BTKi). This approval marks another significant milestone following Relmacabtagene’s previous approvals for R/R large B-cell lymphoma (LBCL) and follicular lymphoma (FL).
The treatment of R/R MCL has always been challenging. Traditional therapies often yield limited long-term results, particularly for patients who have failed BTKi treatment, leaving them with fewer options. The emergence of Relmacabtagene offers a new treatment avenue for these patients. Clinical studies demonstrate that this CAR-T cell therapy can significantly extend progression-free survival (PFS) and overall survival (OS), with a favorable safety profile.
The approval of this new indication not only expands the use of Relmacabtagene in treating R/R MCL but also paves the way for future advancements in MCL treatment. The application of CAR-T cell therapy, especially in high-risk patients, has shown potential in overcoming aggressive lymphomas. For patients with complex genetic characteristics and challenging treatment profiles, Relmacabtagene is undoubtedly a powerful therapeutic option.
Looking ahead, Relmacabtagene is expected to benefit more R/R MCL patients in clinical practice, pushing the boundaries of treatment in this field. As research deepens and new therapies continue to emerge, Relmacabtagene is poised to play an increasingly important role in improving patient outcomes and extending survival, offering new hope to R/R MCL patients worldwide.
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in Chinafor preliminary evaluation!
WhatsApp: +86137 1795 9070
(Http://wa.me/+8613717959070)
Email: doctor.huang@globecancer.com
#BloodCancerTreatment #CAR_TCellTherapy #CancerBreakthrough #Immunotherapy #BCMACART #MedicalAdvancements #CancerSurvivorship #ChinaMedicalInnovation #MCL #RRMCL #HopeForCancerPatients #Relmacabtagene #MCLTreatment #CancerBreakthrough #ChinaApproval #CAR_TCellTherapy #LymphomaTreatment #CancerInnovation #HematologyAdvances
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China’s Fully Human BCMA CAR-T Therapy: Bringing New Hope for High-Risk Relapsed/Refractory Multiple Myeloma
China’s Fully Human BCMA CAR-T Therapy: Bringing New Hope for High-Risk Relapsed/Refractory Multiple Myeloma

Mutiple Myeloma
Multiple Myeloma (MM) is a complex and aggressive cancer originating from plasma cells, posing significant treatment challenges due to its resistance to therapy. For patients with high-risk MM, especially those who experience relapse after multiple lines of treatment, finding an effective therapy is critical. Recently, Chinese medical team successfully treated a case of high-risk relapsed/refractory MM (RRMM) using fully human BCMA CAR-T cell therapy, offering new hope for such difficult cases.
**Case Overview**
The patient, a 56-year-old woman, presented with anemia during a routine check-up and was later diagnosed with MM. Despite undergoing several treatment regimens, including VRd (bortezomib, lenalidomide, dexamethasone) and Dara-DECP followed by autologous stem cell transplantation (ASCT), the disease continued to progress, with extramedullary disease (EMD) manifestations complicating the case.
**Challenges and Treatment Journey**
After initial treatments failed to achieve long-term remission, the patient’s condition worsened, with new lesions detected in the pancreas and multiple subcutaneous nodules indicating possible metastasis. Given the aggressive nature of the disease and the presence of EMD, which is associated with a poor prognosis, the medical team opted for a BCMA-targeted CAR-T cell therapy using Iquarense (Ikaros CAR-T), the first CAR-T product approved for MM treatment in China.
**CAR-T Therapy and Results**
Following pre-conditioning with a fludarabine-cyclophosphamide (FC) regimen, the patient received the BCMA CAR-T therapy. The treatment was well-tolerated, with only mild cytokine release syndrome (CRS) observed. Remarkably, within three months post-treatment, PET-CT scans showed no signs of active disease, and the patient achieved a complete response (CR), which has been sustained for eight months.
**Significance and Future Implications**
This case highlights the potential of BCMA CAR-T therapy as a powerful option for patients with high-risk, relapsed/refractory MM, particularly those with EMD. The successful outcome not only provides new hope for patients facing similar challenges but also contributes valuable insights for future treatment strategies. Iquarense, with its low immunogenicity and prolonged persistence in the body, represents a promising advance in the fight against this formidable disease.
For patients with high-risk MM, it is crucial to consider genetic factors, treatment response, and overall disease dynamics when selecting a therapeutic approach. As this case demonstrates, BCMA CAR-T therapy offers a viable path forward, particularly for those with limited options due to disease progression or EMD.
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
(Http://wa.me/+8613717959070)
Email: doctor.huang@globecancer.com
#BloodCancerTreatment #CAR_TCellTherapy #CancerBreakthrough #Immunotherapy #BCMACART #MedicalAdvancements #CancerSurvivorship #ChinaMedicalInnovation #HopeForCancerPatients
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Multiple Myeloma Solution ** Breakthroughs and Hope in Blood Cancer Treatment**
Multiple Myeloma Solution
** Breakthroughs and Hope in Blood Cancer Treatment**

Mutiple Myeloma
Multiple myeloma is a malignant blood cancer caused by the abnormal proliferation of plasma cells in the bone marrow, often affecting the bones, kidneys, and various organs and systems. With the advancement of modern medical technology, the understanding and treatment of multiple myeloma have greatly improved, bringing unprecedented hope to patients.
### Causes and Risk Factors of Multiple Myeloma
The causes of multiple myeloma are complex, typically involving multiple factors such as genetic mutations, immune system abnormalities, exposure to harmful chemicals, viral infections, and genetic predisposition. Additionally, lifestyle and environmental factors like smoking, obesity, and certain occupational exposures are also believed to increase the risk of developing the disease. With an aging population, the incidence of multiple myeloma is on the rise.
### Evolution of Treatment: Traditional and Innovative Approaches
Although multiple myeloma was once considered a difficult-to-treat disease, recent advancements in treatment methods have made significant progress. Traditional treatments include chemotherapy, radiation therapy, and bone marrow transplantation, which have extended patients’ survival to some extent but come with limited efficacy and significant side effects.
However, with advances in medical technology, new treatment options have emerged. Monoclonal antibodies have played a key role in treating multiple myeloma, targeting cancer cells precisely while reducing harm to healthy cells. Antibody-drug conjugates take this a step further by delivering chemotherapy drugs directly to cancer cells, enhancing efficacy while minimizing side effects.
Small molecule targeted drugs represent another breakthrough. These drugs inhibit specific genes or proteins in cancer cells, preventing their growth and spread. For example, BCL-2 inhibitors and protein kinase inhibitors have shown promising results in clinical trials, offering more treatment options.
### Immunotherapy: Leading the Future of Hope
Immunotherapy is becoming increasingly important in the treatment of blood cancers, especially in the field of multiple myeloma. By activating the patient’s own immune system to attack cancer cells, immunotherapy holds great promise. Among the most forward-looking therapies is CAR-T cell therapy. This treatment involves genetically modifying a patient’s T cells to recognize and kill cancer cells, and it has achieved remarkable results in multiple myeloma patients.
In recent years, China has made significant progress in the research and application of immunotherapy. Particularly in the field of blood cancers, China has developed a comprehensive treatment system with established protocols and consensus. The BCMA-targeted CAR-T cell therapy has shown deep and lasting efficacy in treating multiple myeloma, greatly improving patients’ disease-free survival rates. For instance, China’s fully human BCMA-targeted CAR-T product, Equecabtagene Autoleucel, has demonstrated the best data among CAR-T products for multiple myeloma, with a complete remission rate of 82.4%.
### Looking Ahead
With the continuous emergence of new drugs and therapies, the treatment of multiple myeloma is moving towards individualized and precision medicine. Patients not only experience extended lifespans but also regain their quality of life, returning to normalcy in their everyday lives and work. As medical advancements continue, there is reason to believe that multiple myeloma will no longer be an incurable disease, and every patient will be able to embrace a brighter future.
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070 (Http://wa.me/+8613717959070)
Email: doctor.huang@globecancer.com
#BloodCancerTreatment #CAR_TCellTherapy #CancerBreakthrough #Immunotherapy #BCMACART #MedicalAdvancements #CancerSurvivorship #ChinaMedicalInnovation #HopeForCancerPatients
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Иностранные пациенты ищут лечение: Лучшая терапия CAR-T в Китае! Первый российский пациент с множественной миеломой успешно очистил опухоль и достиг CR!
**Иностранные пациенты ищут лечение: Лучшая терапия CAR-T в Китае! Первый российский пациент с множественной миеломой успешно очистил опухоль и достиг CR!**

В течение пяти лет после постановки диагноза высокорисковой множественной миеломы, господин Ф. испытывал невыносимые страдания. Несмотря на различные схемы химиотерапии и два пересадок, ему не удавалось полностью избавиться от кошмара непредсказуемых рецидивов и прогрессирования опухоли. К счастью, к концу 2023 года его состояние временно стабилизировалось. В сопровождении ведущих гематологов из России, господин Ф. отправился в Китай для прохождения терапии CAR-T, достигнув оптимальных результатов с полным исчезновением опухоли и CR (полная ремиссия).
Достигнуто CR! В феврале 2024 года господин Ф. и его семья успешно вернулись в Россию, чтобы заново начать счастливую жизнь.
(Примечание: Для защиты конфиденциальности пациента в тексте использованы вымышленные имена, а также немного изменены медицинские данные и процесс лечения.)

**Обычная боль в спине оказалась высокорисковой множественной миеломой**
Господин Ф., 63 года, занимает должность заместителя директора технического колледжа в Санкт-Петербурге, Россия. У него успешная карьера и счастливая семья: жена, один сын и две дочери. За несколько месяцев до Рождества 2017 года господин Ф. испытывал усиливающуюся боль в спине, с сильной болью в поясничной области. В сопровождении семьи он отправился в больницу для тщательного обследования. Окончательный диагноз оказался множественной миеломой, с остеолитическими поражениями в более чем трёх участках. Врач сообщил господину Ф., что на основе всесторонней оценки различных результатов анализов его миелома классифицируется как высокорисковая (IgG-тип, стадия DS-IIIА, стадия ISS-II), что делает её трудной для лечения, склонной к рецидиву и с плохим прогнозом. Это ужасное известие пришло настолько внезапно, что вся семья погрузилась в шок и панику. В интервью господин Ф. сказал: “В китайской культуре Конфуций говорил: ‘Когда Небеса собираются возложить на человека великую ответственность, они всегда сначала расстраивают его дух’, рассматривая это как жизненное испытание. В западной культуре это считается наказанием Бога, испытанием, которое нужно пережить, и исход полностью зависит от того, как человек справляется с этими трудностями. Я должен выбрать борьбу!”
**Рецидив и прогрессирование: неизбежный кошмар для пациентов с множественной миеломой**
После двух дней шока и паники господин Ф. решил собраться с силами и найти лучшее лечение для борьбы с опухолью. Господин Ф. и его семья консультировались со многими врачами и клиниками в Испании, Мюнхене и Хайдельберге. Узнав, что терапия CAR-T обладает замечательными целебными свойствами при злокачественных заболеваниях кроветворной системы, они почувствовали луч надежды, который вскоре угас. В то время терапия CAR-T была доступна в очень немногих странах, преимущественно в развитых, и астрономическая стоимость почти в миллион долларов делала её недоступной даже для зажиточного господина Ф. Чтобы как можно быстрее контролировать заболевание, в начале 2018 года господин Ф. прошел традиционное лечение по схеме CVD в России, а затем первую аутологичную трансплантацию костного мозга, достигнув частичной ремиссии (PR). К сожалению, всего через десять месяцев его множественная миелома рецидивировала. После перехода на схему второй линии химиотерапии, он прошел вторую аутологичную трансплантацию костного мозга. Несмотря на самое агрессивное лечение и пережитые опасные для жизни побочные эффекты, опухолевые очаги остались в его теле, как бомба замедленного действия, которая могла взорваться в любой момент. Хотя поддерживающая терапия временно стабилизировала заболевание, господин Ф. и его семья оставались настороже. Они знали, что высокорисковая миелома, скорее всего, вновь рецидивирует и прогрессирует в кратчайшие сроки. Если заболевание снова ухудшится, они окажутся в отчаянной ситуации без доступных методов лечения.

**Глобальное сравнение показывает, что лучшая CAR-T терапия находится в Китае!**
Чтобы полностью избавиться от рака и избежать кошмара постоянных рецидивов и ухудшений, через пять лет господин Ф. и его семья снова начали искать возможность излечения с помощью CAR-T терапии по всему миру. Они провели обширное исследование уже представленных на рынке CAR-T терапий и проконсультировались с множеством больниц и экспертов в Европе, включая Индию и Израиль. К его удивлению, за эти пять лет в этих странах не произошло значительных улучшений. Почти каждый эксперт упомянул, что китайские больницы достигли огромных успехов в области CAR-T, превзойдя даже Европу и США. Особенно в случае множественной миеломы, 30 июня 2023 года Китай представил первую в мире полностью человеческую BCMA CAR-T терапию – Икиоленс. Господин Ф. и его лечащий врач немедленно связались с Международной больницей Цзяхуэй в Шанхае для проведения первоначальной удалённой консультации. Больница Цзяхуэй, являясь онкологическим центром международного уровня с профессиональной мультидисциплинарной MDT командой, провела предварительную оценку. Они пришли к выводу, что план лечения Икиоленс (BCMA CAR-T) будет наиболее эффективным, разработали комплексный план и ответили на все вопросы и опасения господина Ф. и российских экспертов, в конечном итоге достигнув согласия по плану лечения.

Профессор Ли Хуа, директор онкологического центра Международной больницы Цзяхуэй, и его команда российских экспертов-гематологов обсудили план лечения господина Ф. Несмотря на то, что господин Ф. был несколько осведомлен о достижениях китайской медицины, он не мог представить, что здравоохранение Китая превзошло уровень развитых западных стран. Поэтому в ноябре 2023 года лечащий врач господина Ф., известный российский гематолог, сопровождал его в Китай для оценки на месте возможности применения китайской CAR-T терапии. Их первой остановкой по прибытии в Китай была встреча с международной мультидисциплинарной командой больницы Цзяхуэй и осмотр медицинской среды, соответствующей международным стандартам ухода.
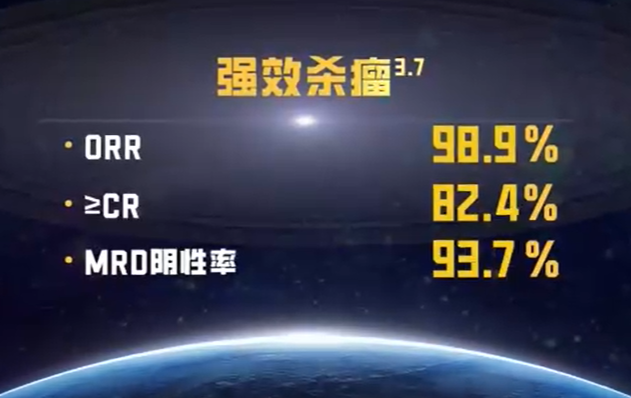
Профессор Ли Хуа, директор онкологического центра Международной больницы Цзяхуэй, и его команда подробно рассказали о плане лечения CAR-T. По сравнению с традиционными методами лечения, CAR-T имеет уникальные преимущества. Во-первых, это высоко персонализированное лечение, которое с помощью генной инженерии регулирует иммунную систему пациента, обеспечивая более точную реакцию на лечение рака. Во-вторых, CAR-T демонстрирует значительно более высокую эффективность в сложных случаях, чего невозможно достичь традиционными методами лечения. Икиоленс имеет общий уровень ответа почти 99% и уровень полного ответа до 82,4%. Что еще более важно, полностью человеческий CAR-T не только поддерживает качество жизни пациента, но и обеспечивает более длительный эффект лечения. Кроме того, однократная инфузионная терапия позволяет достичь быстрых и эффективных результатов, снижая боль и нагрузку в процессе лечения и давая надежду на излечение.

Второй остановкой для господина Ф. и российских экспертов стало посещение производственного завода первой в мире полностью человеческой BCMA CAR-T терапии, Икиоленс. Господина Ф. и российских экспертов поразило то, что эта выдающаяся терапия была полностью разработана в стране, включая независимую разработку лентивирусного вектора. Завод обладает полной платформой для разработки и собственной производственной базой.

В конце концов, господин Ф. развеял все свои сомнения и убедился, что китайское полностью человеческое решение BCMA CAR-T является мировым лидером. Он решил пройти лечение в Китае.
Полное очищение от опухолей, поздняя стадия миеломы достигла CR!
В декабре 2023 года, за несколько дней до очередного Рождества спустя пять лет после постановки диагноза, господин Ф. и его семья прибыли в Китай, полные надежд на излечение, чтобы официально начать свой путь с CAR-T. В международной больнице Цзяхуэй в Шанхае господин Ф. прошел тщательное обследование и после выполнения всех требований успешно прошел аферез, процесс сбора Т-клеток. Затем эти клетки были доставлены обратно на фабрику в течение 24 часов для создания точно направленного оружия против рака, специально для господина Ф.
20 января 2024 года он получил лимфодеплетирующую химиотерапию с флударабином и циклофосфамидом.
25 января 2024 года CAR-T клетки, несущие надежду всей семьи господина Ф., были доставлены ему командой аптеки с использованием профессиональной холодовой цепи логистики. После многократных проверок, распаковки и активации небольшой пакетик молочно-белой жидкости был введен обратно в организм господина Ф. Врачи объяснили, что эта жидкость содержит сотни миллиардов Т-клеток “специального назначения”, которые, попав в организм, начнут интенсивную зачистку, полностью уничтожая клетки миеломы.
Процесс переливания прошел гладко. Что глубоко тронуло господина Ф. и его семью, так это то, что каждый раз, когда он чувствовал беспокойство, медицинская команда держала его за руку, вселяя в него уверенность и силу.
Хотя после переливания возник легкий синдром высвобождения цитокинов (CRS), что является обычным явлением после терапии CAR-T, медицинская команда больницы Цзяхуэй имела большой опыт в лечении этого состояния. Благодаря симптоматическому лечению симптомы улучшились примерно за неделю. На 14-й день после переливания оценка показала, что CAR-T-клетки эффективно расширились в организме господина Ф., достигнув 10^9.
Господин Ф. сказал, что, в отличие от двух болезненных трансплантаций, несмотря на то, что он знал о побочных эффектах терапии CAR-T, медицинская команда четко информировала его о возможных реакциях на разных этапах и заверила, что медицинский персонал немедленно их устранит. В результате он не испытывал никаких беспокойств или страхов.
Перед Китайским Новым годом в 2024 году господин Ф. был выписан и вернулся в Россию для дальнейшего наблюдения в своей стране. Профессор Ли Хуа сказал: “Результаты лечения соответствовали нашим ожиданиям, с значительными улучшениями в ключевых показателях, таких как сывороточный белок M, свободные легкие цепи и симптомы болезни. Все функциональные состояния улучшились.”
В мае 2024 года результаты ПЭТ-КТ показали, что очаги господина Ф. исчезли, и он наконец достиг полной ремиссии (CR). Это означает, что после шестилетней борьбы с множественной миеломой он наконец одержал победу!
Яркое будущее: Китай станет лучшим местом для лечения пациентов с запущенной миеломой!
Сейчас господин Ф. не только возобновил свою счастливую и полноценную жизнь, но и он с женой вернулись к своим любимым профессиям. В настоящее время он занимает должность генерального директора Центра образования в области минеральных ресурсов ЮНЕСКО.
Господин Ф. сказал: “Когда я приехал в Китай и увидел медицинские условия и лично испытал лучшее лечение, я почувствовал глубокую гордость за Китай. К сожалению, Россия пока не может достичь такого уровня ухода. Однако я привезу свои впечатления из Китая в Россию и поделюсь ими. Медицинская система Китая является одной из лучших в мире, и выбор приехать в Китай был абсолютно правильным решением!”

В интервью жена господина Ф. сказала: “Хотя мы уже вышли на пенсию, у нас обоих есть очень важная работа. Это лечение CAR-T в Китае дало нам очень ценный опыт. У нас появилось больше времени, чтобы выслушивать мысли друг друга, поддерживать друг друга и вместе преодолевать трудности.”

Профессор Ли Хуа, директор Международного онкологического госпиталя и заведующий отделением онкологии, сказал в интервью:
“Клинические исследования терапии CAR-T начались в Китае относительно рано. Особенно с 2020 года количество клинических испытаний CAR-T в Китае значительно превысило количество испытаний в США, что дает нам богатый опыт от лабораторных исследований до клинического применения.
Кроме того, лечение CAR-T в Китае более экономически эффективно, предоставляя пациентам более конкурентоспособный вариант.
Самое важное, что в Китае достаточно мощностей для коммерческого производства высокого качества. Пациенты могут быстро получить лечение без ожидания, что крайне важно для больных злокачественными заболеваниями крови, которым требуется своевременное лечение.”
Профессор Ли Хуа, главный врач и главный онколог Международной онкологической больницы Цзяхуй,
Появление CAR-T клеточной терапии принесло новую надежду раковым пациентам по всему миру. Китайская CAR-T терапия отличается высокой эффективностью, хорошей безопасностью, низкой стоимостью и быстрым изготовлением, что дает ей лидирующие преимущества. Несколько международных больниц открыли зеленые коридоры для зарубежных пациентов, что делает Китай лучшим местом лечения для пациентов с множественной миеломой по всему миру!

Использованная литература:
[1] Yuting Yan, et al. Blood Adv. 2019 Oct 8;3(19):2895-2904.[2] 2023 IMS. P-290.
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Redefining Hope for High-Risk RRMM Patients Worldwide: A Singaporean Patient`s Journey to China for Groundbreaking CAR-T Therapy
Redefining Hope for High-Risk RRMM Patients Worldwide: A Singaporean Patient`s Journey to China for Groundbreaking CAR-T Therapy

Multiple Myeloma
