Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese Research Team New Treatment Shows Promise: CD7 CAR-T Cell Therapy for Relapsed/Refractory AML
Chinese Research Team New Treatment Shows Promise: CD7 CAR-T Cell Therapy for Relapsed/Refractory AML

A recent clinical trial has unveiled a new prospect for treating relapsed/refractory acute myeloid leukemia (AML). The study delved into the safety and efficacy of a natural selection CD7 CAR-T cell (NS7 CAR-T cell) in treating CD7+ relapsed/refractory AML patients.
The research involved 10 cases of CD7+ relapsed/refractory AML, where 30% of AML patients expressed CD7. Following treatment with NS7 CAR-T cell infusion, the results showed that within 4 weeks post-infusion, 70% of patients achieved complete bone marrow remission (CR), with 6 patients achieving a deep remission at the level of minimal residual disease (MRD). Notably, the loss of CD7 was identified as a primary reason for non-response in some patients.
The study also indicated that the majority of patients (80%) experienced mild cytokine release syndrome (CRS) post-infusion, with no observed neurotoxicity.
Furthermore, for select patients, continuing with a second allogeneic hematopoietic stem cell transplantation post NS7 CAR-T cell treatment resulted in promising long-term outcomes. However, further exploration is required for patients who did not undergo consolidative transplants to enhance treatment efficacy.
Professor Lu Peihua(Lu Daopei Hospital) remarked that this study highlights the potential of CD7 CAR-T cells as a bridging therapy before transplantation, demonstrating preliminary therapeutic efficacy for relapsed/refractory AML patients. Nevertheless, a comprehensive evaluation of its efficacy requires a larger sample size and longer-term follow-up.

This preliminary study represents a significant advancement in the field of AML treatment, but collaborative efforts and more research are still necessary. Looking ahead, we anticipate further studies and treatment outcomes to bring more beneficial therapeutic options for the long-term survival of patients, supported by a broader patient cohort and collaborative efforts from the medical community.
The outlook for this CD7 CAR-T cell therapy is promising, especially for AML patients who have undergone various treatments and allogeneic hematopoietic stem cell transplants. We look forward to more in-depth research and treatment outcomes in the future, offering more beneficial treatment choices for long-term survival among patients.
(source: ClinicalTrials.gov registration number NCT04938115
Preliminary results published by Professor Lu Peihua and their team in a top-tier journal)

#AMLResearch #CancerTreatment #CARTTherapy #Leukemia #MedicalBreakthrough #Cancer #AML #Immunotherapy #ClinicalTrials #BloodCancer #PatientCare #HealthcareAdvances #PrecisionMedicine #ScienceAndHealth #MedicalScience #CancerSurvivor #AMLWarriors #ResearchProgress #CancerFree #HopeInTreatment #acutemyeloidleukemia
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

A significant breakthrough in Chinese solid tumor CAR-T therapy, resulting in complete remission for pancreatic cancer patients.
Chinese pharmaceutical company SciCoMed announced a significant breakthrough in treating metastatic pancreatic cancer with its independently developed CAR-T cell therapy targeting Claudin18.2, known as CT041. Pancreatic cancer, often termed the “king of cancers,” presents a significant challenge with low survival rates. However, CT041’s two representative cases have garnered attention: two patients with refractory pancreatic cancer, who failed multiple lines of treatment, received CT041 therapy. The results showcased a substantial reduction in tumor lesions and, in some cases, complete disappearance, offering a glimmer of hope for pancreatic cancer patients.

In the case of a 58-year-old female patient, lung metastases significantly decreased post CT041 treatment. Similarly, a 75-year-old female patient achieved complete remission by the fourth week post-treatment, maintaining remission to date. These successful cases validate the remarkable efficacy of CT041 for refractory pancreatic cancer, sparking interest and anticipation among international experts.
Moreover, CT041 has garnered recognition for its efficacy in the field of digestive system tumors. As the world’s first CAR-T cell therapy targeting Claudin18.2, it demonstrates promising prospects in treating digestive system tumors. Research data illustrates that among 37 patients, 83.3% experienced tumor regression, with an objective response rate of 48.6%. Additionally, CT041 displayed significant effectiveness in late-stage gastric and pancreatic cancer patients, offering hope for future treatments.
The rapid development of CAR-T therapy in China involves over 20 companies contributing significantly. CT041’s success signifies China’s progress toward becoming a global leader in CAR-T therapy. Moving forward, scientists will continue efforts to enhance efficacy and reduce side effects, providing a ray of hope for more late-stage cancer patients.
The new hope for pediatric patients with relapsed/refractory B-cell acute lymphoblastic leukemia (R/R B-ALL) boasts an overall survival rate of up to 96%.
The new hope for pediatric patients with relapsed/refractory B-cell acute lymphoblastic leukemia (R/R B-ALL) boasts an overall survival rate of up to 96%.

Recently, CAR-T cell therapy targeting B-cell malignancies has encountered a series of inquiries and challenges, particularly concerning discussions on CAR-T cell-related toxicity, resistance, antigen escape, and limitations in persistence. However, a groundbreaking concept addressing relapse in patients after CAR-T cell therapy has been introduced for the first time: a sequential approach involving distinct targeted CAR-T cell therapies.
Within this approach, CD19 CAR-T cell therapy has demonstrated the ability to achieve complete remission in 60% to 90% of relapsed or refractory acute B-cell lymphoblastic leukemia patients. By experimenting with different combinations and sequential administration strategies of B-cell antigen-targeted CAR-T cell therapies, there’s potential to prevent tumor antigen escape and prolong the persistence of CAR-T cells.
Preliminary clinical trials have provided initial support for this concept, notably a phase II clinical trial aimed at assessing the efficacy of sequential CD19 and CD22 CAR-T cell therapy. Its findings revealed a 79% event-free survival rate, an 80% sustained remission rate, and an impressive 96% overall survival rate among patients receiving targeted doses in sequential therapy. Encouragingly, the overall safety of this sequential therapy appeared manageable, providing long-term survival benefits for children with relapsed or refractory acute B-cell lymphoblastic leukemia.
However, the limitations of antigen escape and limited persistence after CAR-T cell therapy persist. Addressing these challenges, researchers have proposed the hypothesis of sequential administration of CAR-T cell products targeting different antigens, aiming to maintain the persistence of CAR-T cells.
The results of this phase II clinical trial indicate that administering CD22 CAR-T cell therapy following CD19 CAR-T cell infusion can result in longer-lasting remission effects for pediatric patients with relapsed or refractory B-cell acute lymphoblastic leukemia, achieving an 80% sustained remission rate over 18 months and an impressive 96% overall survival rate. Importantly, the overall safety of this sequential therapy is uplifting, providing long-term survival benefits for this specific patient population.
In summary, this study presents groundbreaking evidence for new strategies and directions in CAR-T cell therapy. Despite existing limitations, this therapy demonstrates significant potential in treating uncontrollable acute B-cell lymphoblastic leukemia, potentially offering more enduring treatment effects and long-term survival benefits for these patients. This achievement points towards a viable path for the future development of cell therapies.
This Phase 2 trial, conducted at Beijing GoBroad Boren Hospital in China, enrolled pediatric patients aged 1–18 years diagnosed with relapsed or refractory B-cell acute lymphocytic leukaemia (ALL) showing CD19 and CD22 positivity exceeding 95%.

If a child unfortunately gets leukemia, should CAR-T Therapy be considered?
In recent years, CAR-T therapy has gained traction in treating leukemia. A quick search yields numerous articles, many of which describe the miraculous effects of CAR-T.
Is the efficacy of CAR-T therapy really that impressive? Should CAR-T therapy be considered for a child diagnosed with leukemia? Today, let’s unravel these doubts together!
What kind of leukemia patients can undergo CAR-T therapy?
Since most acute leukemia patients are sensitive to chemotherapy, chemotherapy is the preferred initial treatment. Currently, CAR-T therapy is primarily used for refractory and relapsed acute B-cell lymphoblastic leukemia patients.
For cases where 1-2 courses of chemotherapy fail to achieve complete remission (primary refractory), or relapse during chemotherapy, or experience ineffective re-treatment after relapse and re-chemotherapy (refractory relapse), CAR-T therapy is the preferred approach.
Celebrating a decade of being cancer-free for the world’s first leukemia child treated with CAR-T therapy
The world’s first leukemia child cured by CAR-T therapy celebrates 11 cancer-free years
On May 10th each year, Emily Whitehead commemorates the anniversary of her cancer-free survival. This year, she turns 18 and has officially become a nurse, leading a busy and joyful life. With the achievement of this significant milestone, the revolutionary cancer treatment known as CAR-T cell immunotherapy has been officially recognized! She has also become the spokesperson for this epic therapy.
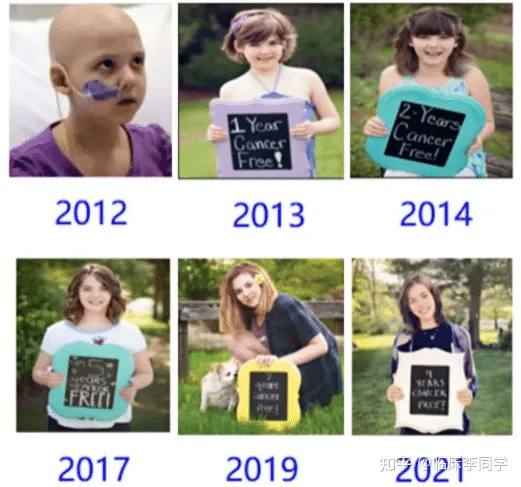
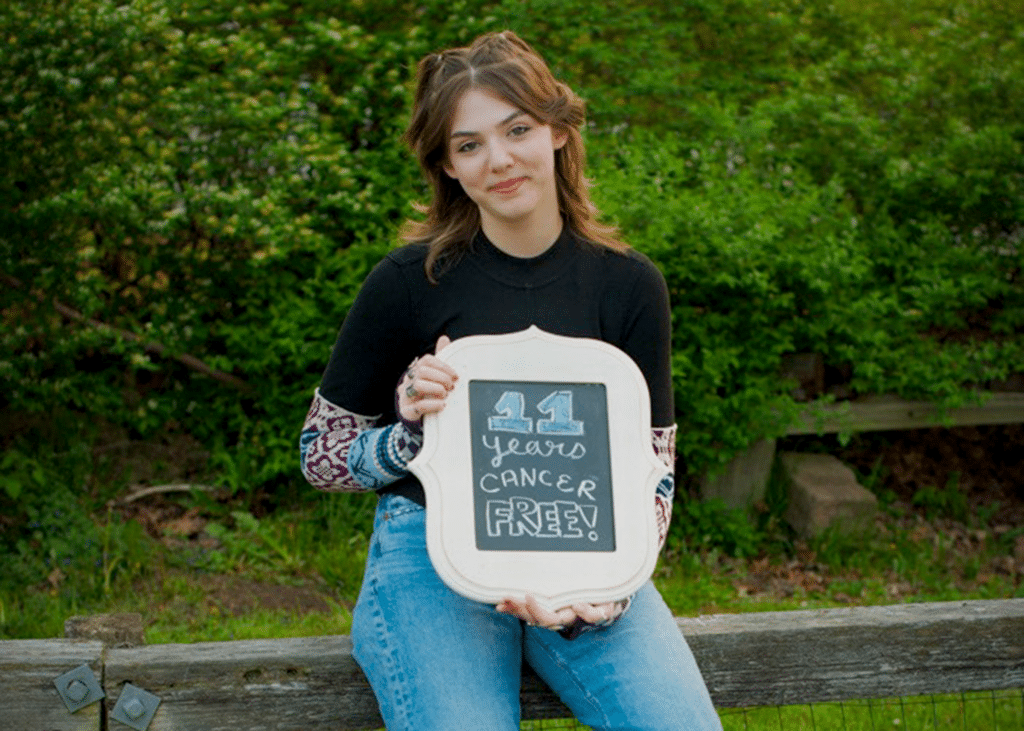
Which CAR-T products are currently on the market?
Since 2017, the approval of the world’s first CAR-T products, Novartis’s Kymriah, and Kite Pharma’s Yescarta by the FDA, has heralded a new era in cell immunotherapy. Presently, there are 10 CAR-T drugs approved for market globally. Additionally, there are over 1,000 CAR-T clinical trials registered worldwide on Clinicaltrials, with nearly 500 projects in mainland China. The primary treatment areas include hematologic malignancies, along with solid tumors such as pancreatic cancer, liver cancer, lung cancer, breast cancer, and colorectal cancer.
More and more people are choosing to seek medical treatment in China. China’s technology in oncology, especially in CAR-T, is now on par with that of the United States. The fundamental reasons why many individuals with related diseases choose China are the high-quality services, pleasant environment, and comparatively affordable prices.

“If you’d like to inquire about the latest cancer-fighting technologies and treatments, you can contact us.”
whatsapp:+8613717959070
