Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

New Advances in Chinese CAR-T Cell Therapy: CD27 Armored BCMA CAR-T Shows Potential in Treating Relapsed Multiple Myeloma
**New Advances in Chinese CAR-T Cell Therapy: CD27 Armored BCMA CAR-T Shows Potential in Treating Relapsed Multiple Myeloma**

Multiple Myeloma
#BCMA #CD27#CARTCellTherapy #RRMM #MM #MultipleMyeloma #CART
Relapsed and refractory multiple myeloma (RRMM) is a challenging blood cancer that poses significant obstacles for patients. Although several BCMA (B-cell maturation antigen) targeting CAR-T cell therapies have been developed, achieving long-term remission and extended survival remains difficult. The recent introduction of CD27-armored BCMA CAR-T—CBG-002—by a Chinese medical team brings new hope for RRMM patients. Based on preclinical data, this therapy shows promising anti-myeloma activity, with Phase I clinical trial results further validating its efficacy and safety.
Research Highlights
CBG-002 is an innovative therapy that enhances traditional BCMA CAR-T by adding a CD27 costimulatory domain. The inclusion of CD27 improves CAR-T cell persistence and activity, contributing to stronger anti-cancer effects. Recently published Phase I research in Cancer Immunology Research indicates that this CD27-armored BCMA CAR-T therapy is not only well-tolerated but also shows notable efficacy in RRMM patients.
The study enrolled 11 RRMM patients, all of whom had undergone three or more prior treatments, with a median age of 54. The results showed that 81.8% of patients experienced only mild cytokine release syndrome (CRS) and no severe neurotoxicity. Even at a low dosage level (1-3×10^6 CAR-T cells/kg), CBG-002 achieved a high overall response rate (ORR) of 81.8%, with 45.5% of patients reaching stringent complete remission.
Future Outlook
The Phase I study of CBG-002 not only confirmed its efficacy but also demonstrated advantages in production time and cost. Compared to current products on the market, CBG-002 has a shortened production cycle of just 10 days, making it a timely and affordable treatment option for more patients. Amid global progress in CAR-T cell therapies, the innovation by this Chinese medical team positions it as a significant force in advancing cancer research worldwide.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#MultipleMyeloma #CancerResearch #Immunotherapy #ChinaBiotech #Oncology
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Why Are More and More International Patients Coming to China for CAR-T Cell Therapy?
Why Are More and More International Patients Coming to China for CAR-T Cell Therapy?

CARTtherapy
#CARTtherapy #InternationalPatient #CART #Multiplemyeloma #lymphoma #leukemia
In recent years, CAR-T cell therapy, as an innovative cancer treatment, has rapidly gained widespread attention globally. China, as a leading country in the CAR-T treatment field, has attracted an increasing number of patients from Europe and Asia due to its advanced technology, high-quality medical resources, and relatively lower treatment costs. So, why are more international patients choosing to come to China for CAR-T treatment? Here are some key reasons worth noting:
### 1. **Advanced CAR-T Treatment Technology**
Over the past decade, China has made tremendous strides in medical research, particularly in the field of cancer immunotherapy. Not only has China successfully developed various CAR-T therapies, but it has also built a comprehensive treatment protocol and safety monitoring system to ensure efficient and safe treatment for patients. Additionally, China has accumulated extensive experience in managing treatment-related side effects, such as CRS (Cytokine Release Syndrome) and neurotoxicity.
Currently, more than 50% of the world’s clinical trials for CAR-T therapy are conducted in China. Of the 11 CAR-T products already on the global market, five are from China. These include products for multiple myeloma (BCMA CAR-T), diffuse large B-cell lymphoma (CD19 CAR-T), and leukemia. Compared to similar products in the U.S. for multiple myeloma, China’s CAR-T products are not only fully humanized but also boast the highest complete remission rates among all available products, reaching 82.4%. The CR data for China’s lymphoma and leukemia CAR-T products are also impressive, at 77.6% and 82.1%, respectively.
### 2. **International Certification and Clinical Trial Opportunities**
Several hospitals and research institutions in China have gained international recognition for their CAR-T therapies. Patients can choose between commercial CAR-T treatments or participate in clinical trials. For some CAR-T treatments that have not yet been approved or are hard to access in Europe or Asia, patients can receive these cutting-edge therapies in China earlier.
Just a few days ago, Chinese scholars’ research on universal CAR-T cell therapy was featured on the cover of *Nature*. Various other studies on Chinese CAR-T therapies are frequently published in major medical journals. For example, a Chinese medical team published a study on dual-target CAR-T in *JAMA Oncology*, showing a 100% stringent complete remission (sCR) rate in high-risk multiple myeloma patients. Additionally, a research team from Zhejiang, China, published clinical data in *Cell Discovery*, highlighting the breakthrough of the fourth-generation anti-CD19 CAR-T cell therapy in treating relapsed/refractory large B-cell lymphoma with exceptional anti-tumor capabilities.
### 3. **Relatively Low Treatment Costs**
Compared to the million-dollar treatment costs in Western countries, CAR-T treatment in China is relatively affordable, often costing just 1/5 to 1/7 of the price in the U.S. For instance, the cheapest CAR-T product for multiple myeloma in the U.S. costs $420,000, and when including hospitalization and other expenses, the total cost can reach around $700,000. In contrast, similar Chinese products, with equal or even better effectiveness, cost only $160,000, and hospitalization and other fees are much lower, often under $10,000. Some treatment options are available for just a few tens of thousands of dollars, and in certain cases, treatment is even completely free.
Patients from Europe and Asia can receive world-class CAR-T therapy in China at a much lower cost, providing an affordable option for those who find the high costs of treatment elsewhere prohibitive. Moreover, a range of one-stop medical services, including hospital visits, translation assistance, transportation, accommodation, and even family care, can significantly enhance the patient experience for international patients.
### 4. **World-Class Expert Teams**
China is home to a group of internationally renowned oncology and immunotherapy experts who have extensive clinical experience and academic achievements in hematological cancers and CAR-T therapy. Patients receive personalized treatment plans designed by leading experts, ensuring that each treatment is precisely tailored to their specific condition, maximizing effectiveness and minimizing risks.
For example, Professor Huang Xiaojun, Director of the Institute of Hematology at Peking University, is one of the world’s pioneers in haploidentical transplantation, having developed China’s haploidentical hematopoietic stem cell transplantation model. His innovative approach has increased the 3-year survival rate of leukemia patients receiving haploidentical transplants from around 20% to approximately 70%, earning him the Distinguished Service Award from the Center for International Blood and Marrow Transplant Research.
### 5. **Efficient and Convenient Medical Services**
China has implemented a 144-hour visa-free transit policy for citizens of 54 countries and mutual visa exemptions with 24 countries. This is a major benefit for international patients seeking treatment in China. Moreover, China’s healthcare system is known for its efficiency and convenience, especially in treating major diseases. Appointment wait times are short, and there is minimal delay in receiving treatment. A “super green channel” is also available for international patients, ensuring quick diagnosis and treatment planning. From initial consultation to online meetings with specialists, the process typically takes less than a week, with hospital admission and treatment beginning within another week.
Compared to the long waiting periods in other countries, China’s efficient medical services significantly improve treatment success rates and patient satisfaction. CAR-T therapy, in particular, is known for its high efficiency. There was an international patient who went from initial consultation to complete remission and a safe return home in less than a month—a remarkable achievement considering CAR-T is a “living” drug that must be custom-prepared using the patient’s own blood cells.
### 6. **Abundant Success Stories**
Many international patients who have received CAR-T therapy in China have achieved remarkable results, with their cancers being effectively controlled or even cured. These success stories not only boost confidence among patients worldwide but also attract more people to China for treatment. By sharing these stories, more patients learn about China’s advantages in CAR-T therapy and choose it as their treatment destination.
For instance, Ethan, a patient from Singapore, achieved complete remission just four weeks after CAR-T treatment and has since recovered very well. There is also the story of a Russian patient with high-risk, late-stage multiple myeloma who received efficient treatment in China at a much more reasonable cost and with a faster treatment process.
### 7. **Conclusion**
In conclusion, as China continues to enhance its technological capabilities in CAR-T cell therapy, more and more patients from around the world are choosing to come to China for this cutting-edge cancer treatment. With its advanced medical technology, international services, and relatively lower treatment costs, China is becoming an important destination for cancer patients seeking treatment. If you or a loved one is searching for innovative cancer treatment options, consider CAR-T therapy in China for a chance at recovery!
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can contact us for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CellTherapy #CancerTreatment #Immunotherapy #HealthcareInnovation #MedicalTourism #ChinaHealthcare #CancerSurvivor #PatientStories #MedicalAdvancements #AffordableHealthcare #PersonalizedMedicine #GlobalHealth #HealthJourney #Oncology
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

What Types of Lymphoma Patients Are Suitable for CAR-T Treatment in China?
# What Types of Lymphoma Patients Are Suitable for CAR-T Treatment in China?

Lymphoma
#CART #Lymphoma #DLBCL #LBCL #MedicalTourism #FL #MCL
In recent years, CAR-T cell therapy has sparked a revolution in cancer treatment worldwide, achieving remarkable results, especially in lymphoma care. As one of the global leaders in CAR-T therapy, China has attracted a large number of international patients seeking this cutting-edge immunotherapy. So, what types of lymphoma patients are suitable to travel to China for this advanced treatment?
## Indications: The First Choice for Relapsed or Refractory Lymphoma Patients
CAR-T treatment in China primarily targets patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), and mantle cell lymphoma (MCL). Specific patient groups that are suitable include:
1. **Relapsed DLBCL**: Patients who did not respond to first-line treatments, such as R-CHOP, or relapsed within 12 months after treatment.
2. **Follicular Lymphoma (FL)**: Patients with grade 1-3a FL who have relapsed or are refractory after at least two lines of treatment.
3. **Mantle Cell Lymphoma (MCL)**: Patients whose disease remains difficult to control even after multiple treatments, including BTK inhibitors.
4. Patients whose lymphoma is resistant to traditional treatments or who experience rapid relapse after therapies such as conventional chemotherapy and targeted treatments.
5. More indications include diffuse large B-cell lymphoma (not otherwise specified), DLBCL transformed from follicular lymphoma, primary mediastinal large B-cell lymphoma, and high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangement, as well as grade 3b follicular lymphoma.
CAR-T therapy offers new hope to these patients by targeting the CD19 antigen on the surface of tumor cells.
## Physical Condition: Stable Health is Key
While CAR-T therapy has shown significant efficacy, the potential for serious side effects, such as **cytokine release syndrome (CRS)** and **neurotoxicity**, means that patients must be in stable health to tolerate the risks associated with treatment. Suitable patients should meet the following conditions:
– **Good heart and lung function**: The patient’s cardiovascular and respiratory health should be adequate to handle the stress of treatment.
– **Normal liver and kidney function**: Liver enzymes (such as ALT) and creatinine levels should be within normal ranges to ensure that the patient can properly eliminate toxins from the body.
– **Stable immune system**: Patients should not have severe infections or immunosuppressed conditions, as CAR-T therapy can further weaken the immune system, increasing the risk of infection.
Additionally, patients need to achieve a performance status score of 0-1 on the ECOG scale, indicating that they have good functional ability and can handle daily activities independently.
## Pre-Treatment Testing: Ensuring Proper Antigen Expression
CAR-T therapy targets specific antigens (such as CD19) on the surface of tumor cells, so patients’ cancer cells must express these antigens for treatment to be effective. If tests show that the tumor cells do not express the relevant antigens, CAR-T therapy will not work effectively.
## Costs and Resources: The Advantages of CAR-T in China
Compared to Western countries, CAR-T therapy in China is relatively affordable, usually costing between hundreds of thousands to one million RMB, whereas in the U.S., CAR-T treatment can cost several hundred thousand dollars. For patients with the financial ability but who find it difficult to bear the high costs of treatment in the West, China offers a significant cost advantage.
Moreover, China boasts several top-tier hospitals with established CAR-T treatment programs, such as leading cancer centers in Beijing, Shanghai, and Guangzhou. These hospitals have extensive clinical experience, offering international patients convenient access to treatment.
## Contact Us:
The Advanced Medicine in China team has a wealth of connections with doctors and hospitals, enabling us to quickly help you find the most suitable hospital and expert team for your specific needs. In China, we provide a fast-track option for CAR-T treatment.
## Conclusion
With its cost advantages, advanced medical resources, and broad indications, CAR-T therapy in China has attracted a large number of international lymphoma patients. For those who have failed traditional treatments, are in good physical condition, and have sufficient financial means, CAR-T treatment in China is an ideal choice. If you or someone close to you meets these conditions, consider coming to China for world-leading CAR-T therapy, and open the door to new hope in the fight against cancer.

 To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CancerTherapy #CARTinChina #RelapsedLymphoma #RefractoryLymphoma #FollicularLymphoma #MantleCellLymphoma #Immunotherapy #CancerRevolution #AdvancedCancerCare #CD19Targeting #CancerHope #CancerCare #ChineseHospitals #GlobalHealth #CancerSurvivors #CARTSuccess #MedicalInnovation
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

**CAR-T Therapy: A New Hope for Effective Immunotherapy or an Expensive Challenge?**
**CAR-T Therapy: A New Hope for Effective Immunotherapy or an Expensive Challenge?**
As an innovative immunotherapy, CAR-T therapy has gradually become available in China in recent years, attracting significant attention from patients. Although it is dubbed the “million-dollar therapy” due to its high cost, experts emphasize that CAR-T is not a conventional drug but a “living drug.” Its effectiveness is confirmed, but the outcome largely depends on the skill and experience of the operator. Like a horse-riding competition, the handler must fully understand and control this “good horse.” Early data show promising results, and while the overall treatment cost remains high, its efficacy is undeniable.
To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#cart #carttherapy #CAR_T #leukemia #lymphoma #cancer #tumor #bloodcancer
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

**China’s CAR-T Therapy Brings Complete Remission for Primary Refractory Multiple Myeloma Patient**
**China’s CAR-T Therapy Brings Complete Remission for Primary Refractory Multiple Myeloma Patient**

Multiple Myeloma
#MultipleMyeloma #CARTtherapy #BloodCancer #CompleteRemission #CART
Multiple Myeloma (MM) is a malignant blood disorder characterized by abnormal proliferation of plasma cells, which causes damage to vital organs. Patients with high-risk cytogenetic abnormalities like 17p deletion, t(4;14) translocation, and t(14;16) translocation face an even worse prognosis. While new drugs like proteasome inhibitors and autologous stem cell transplantation have improved MM treatment outcomes, relapsed/refractory multiple myeloma (RRMM) continues to be a challenge due to its aggressive nature and tendency to relapse. Despite the use of traditional therapies, many patients either fail to respond or cannot tolerate further treatment due to underlying health issues.
CAR-T cell therapy, a novel immunotherapy that re-engineers the patient’s own T cells to attack cancer cells, has shown remarkable results in RRMM patients. It offers an alternative for patients who have poor tolerance to conventional treatments, presenting a safer and more effective option. In particular, Equecabtagene Autoleucel, a CAR-T therapy targeting the BCMA antigen on myeloma cells, has demonstrated success in treating patients unresponsive to frontline therapies.
### Case Study: High-Risk MM Patient Achieves Complete Remission
A 45-year-old male patient diagnosed with high-risk multiple myeloma in June 2023 sought further treatment at our hospital after failing to respond to chemotherapy. He had a history of coronary artery disease, diabetes, hypertension, and kidney artery stenosis, making traditional therapies less effective and risky.
Upon evaluation, the patient’s tests indicated κ-type M protein and abnormal free light chains (FLC-κ of 2410.5mg/L). Imaging scans revealed extensive bone damage caused by multiple myeloma. Genetic testing showed high-risk markers including 17p deletion and 1q21 amplification, further complicating his prognosis.
Given his high-risk status and poor response to standard treatments, the medical team decided to proceed with CAR-T cell therapy using Equecabtagene Autoleucel. After a smooth cell collection and lymphodepletion process, the patient received CAR-T infusion on November 15, 2023.
### Treatment Outcome and Safety
Eight days after infusion, the patient developed a mild fever, indicating cytokine release syndrome (CRS), which was effectively managed with symptomatic treatment. Despite mild kidney dysfunction and temporary blood cell reductions, no severe side effects or neurotoxicities occurred. By day 42, the patient developed a lung infection, which was treated with antibiotics, leading to a full recovery.
At the one-month follow-up, flow cytometry showed no minimal residual disease (MRD), confirming the patient had achieved complete remission (CR) with MRD negativity. This outcome was particularly significant considering the patient’s failure to respond to previous treatments.
### A Promising Future for High-Risk MM Patients
This case highlights the potential of CAR-T cell therapy, particularly Equecabtagene Autoleucel, in achieving deep remission for high-risk multiple myeloma patients who fail to respond to conventional therapies. With ongoing advancements in biomedical technology and immunotherapy, CAR-T treatments offer hope for RRMM patients, providing longer disease-free survival and improved quality of life.
🎉🎉To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#ChinaMedicalAdvances #InnovativeTreatments #Immunotherapy #EquecabtageneAutoleucel #CancerResearch
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

庆祝出院:新生的喜悦 /希望之路 /与癌症抗争 /希望 /CART/多发性骨髓瘤
###庆祝出院:新生的喜悦
经过一段时间的治疗,新加坡患者Teresa在上海嘉会国际医院的CAR-T治疗取得了令人振奋的成果。她的病情完全达到了完全缓解(CR),在这一喜讯的见证下,医院为她举行了一场简短而温馨的庆祝活动。
在庆祝合影中,Teresa与Dr. Vicky Lee及其团队的医生护士们笑容满面,洋溢着胜利的喜悦。照片不仅记录了这份喜悦,也见证了医护人员的辛勤付出和专业精神。Dr. Vicky Lee和团队的每一位成员都是真正的英雄,他们用专业知识和无私奉献帮助Teresa战胜了病魔。
Teresa感慨万千,她说:“这段时间的治疗过程虽然艰辛,但在嘉会医院医生和护士们的悉心照料下,我感受到了无比的温暖与支持。他们的专业和关怀让我充满了信心,最终取得了这样的好结果。”
出院那天,医护人员为Teresa送上了最真挚的祝福。护士们亲切地叮嘱她出院后的注意事项,确保她在回家后的康复过程中能够继续保持良好的健康状态。Teresa感激地与每一位医护人员道别,感谢他们在她最需要的时候给予的关爱和支持。
站在医院门口,Teresa回望这段治疗旅程,心中充满了感激和希望。她知道,正是因为有了这些无私奉献、专业卓越的医护人员,她才能重新拥抱健康,迎接新的生活。
Teresa的康复故事不仅是她个人的胜利,更是嘉会医院全体医护人员共同努力的成果。她深深感谢他们的辛勤付出,并相信未来将会有更多的患者在这里获得新生的希望。
我们将持续关注患者的治疗后续,并跟进报道。
#CART #CARTTherapy #Hopeforpatients #FUCASO #Equecel #MultipleMyeloma #jihuiHospital #Shanghai #ChineseCart #MedicalInnovation #MedicalBreakthrough #CancerTreatment #FullyHumanCART #cancerfight #cancersurvivor #Jiahuihospital
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Redefining Hope for High-Risk RRMM Patients Worldwide: A Singaporean Patient`s Journey to China for Groundbreaking CAR-T Therapy
Redefining Hope for High-Risk RRMM Patients Worldwide: A Singaporean Patient`s Journey to China for Groundbreaking CAR-T Therapy

Multiple Myeloma
#MultipleMyeloma #CAR_Therapy #CancerTreatment #HRMM #MM #RRMM #CART
In the fight against multiple myeloma (MM), the last few decades have seen significant advancements, yet the disease remains notoriously difficult to cure, particularly in patients with relapsed/refractory multiple myeloma (RRMM). These patients face enormous challenges, as their options become increasingly limited after multiple lines of therapy have failed. However, hope has emerged in the form of BCMA CAR-T therapy, offering deep remission and long-term survival for those who had nearly lost hope.
One such case is a 58-year-old woman from Singapore, who after exhausting all available treatments in her home country, found new hope in China`s innovative CAR-T therapy. Diagnosed with MM in May 2021 following a month of severe back pain, she underwent a series of treatments including CD38 monoclonal antibodies, immunomodulatory drugs (IMiDs), proteasome inhibitors (PI), and XPO-1 inhibitors. Unfortunately, these therapies failed to halt the progression of her disease, which had become highly resistant to treatment.
In December 2023, she traveled to Beijing Chaoyang Hospital, Capital Medical University, where Professor Chen Wenming took charge of her case. The patient was diagnosed with high-risk MM (IgG-κ type) and admitted to the hospital on November 25, 2023, for CAR-T therapy.
#### The Treatment Journey: A Detailed Overview
Given the patient’s refractory nature and multiple prior treatments, Professor Chen devised a tailored treatment plan to improve her chances of survival and quality of life. In November 2023, her lymphocytes were collected to prepare the CAR-T cells. During this period, she received two cycles of D-PACE (dexamethasone, cisplatin, doxorubicin, cyclophosphamide, and etoposide) chemotherapy in Singapore to control the extramedullary plasmacytoma.
In February 2024, she returned to Beijing for further evaluation, where her condition was assessed as showing minimal response (MR). She was then administered a lymphodepletion regimen of fludarabine and cyclophosphamide on February 29. The following month, she received a transfusion of BCMA CAR-T cells.
Within five days post-transfusion, the patient developed a fever, which peaked at 39°C. Fortunately, her oxygen saturation and heart rate remained normal, and she was diagnosed with Grade 1 cytokine release syndrome (CRS). After symptomatic treatment, her blood counts recovered by day 15, and she was discharged in stable condition.
Two weeks post-CAR-T therapy, her response was evaluated as a very good partial response (VGPR), and by the two-month mark, her condition had improved to complete remission (CR) with minimal residual disease (MRD) negativity.
#### Insights from Leading Experts
Professor Wee Joo Chng, a specialist in high-risk MM, noted the aggressive nature of the patient’s disease, marked by genetic abnormalities like 1q21+ and t(4;14). Despite the use of multiple potent therapies, including KRd, XVd, and Isa-Pd, the patient’s disease continued to progress rapidly. The emergence of the del(17p) mutation further complicated her prognosis, indicating the need for a novel therapeutic approach.
The FUMANBA-1 study has highlighted the effectiveness of China`s indigenous CAR-T product, Equecabtagene Autoleucel, in achieving deep remission and prolonging survival in RRMM patients. This case demonstrated the therapy`s potential to overcome poor prognostic factors and extend the patient’s survival. Notably, the patient experienced only mild CRS and no immune effector cell-associated neurotoxicity syndrome (ICANS) or infections during treatment. At the two-month follow-up, the patient’s condition had improved to CR with MRD negativity, suggesting that Equecabtagene Autoleucel could be a game-changer for high-risk RRMM patients.
#### A New Frontier in CAR-T Therapy
RRMM patients with double-hit characteristics often experience early relapse and progression, leading to shortened survival times. Traditional therapies, including IMiDs, PIs, and monoclonal antibodies, have failed to overcome these poor prognostic factors, indicating the urgent need for novel treatments. Real-world studies have shown that CAR-T therapy offers comparable progression-free survival (PFS) and overall survival (OS) rates in RRMM patients, regardless of high-risk cytogenetic abnormalities.
The FUMANBA-1 study revealed impressive outcomes for Equecabtagene Autoleucel in RRMM patients, with an overall response rate (ORR) of 98.9% and an MRD negativity rate of 97.8% among CAR-T-naive patients. The CR rate was 82.4%, and 81.7% of patients maintained MRD negativity for over a year.
Globally, four CAR-T products are currently available, and a recent study presented at the 2024 European Society for Blood and Marrow Transplantation (EBMT) compared the short- and long-term efficacy of these therapies. The study’s matching-adjusted indirect comparison (MAIC) analysis revealed that Equecabtagene Autoleucel had a 12-month PFS rate of 94.2%, higher than the 75% observed with Ciltacabtagene autoleucel (CARTITUDE-1 study). Furthermore, the 12-month sustained MRD negativity rate for Equecabtagene Autoleucel was 100%, compared to 53.1% for Ciltacabtagene autoleucel.
These findings suggest that Equecabtagene Autoleucel, a Chinese-developed BCMA CAR-T therapy, offers superior long-term efficacy compared to its U.S. counterpart. As the global community celebrates the first anniversary of its approval, Equecabtagene Autoleucel continues to bring hope to RRMM patients worldwide, further solidifying China’s leading role in the field of cellular therapy.

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp:+8613717959070
Email: doctor.huang@globecancer.com
#ChinaMedicalInnovation #GlobalHealth #MMResearch #PatientCare #InnovativeMedicine #CellTherapy #MedicalBreakthrough
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese CAR-T Therapy: A New Frontier in Treating Systemic Lupus Erythematosus
Chinese CAR-T Therapy: A New Frontier in Treating Systemic Lupus Erythematosus

Systemic Lupus Erythematosus
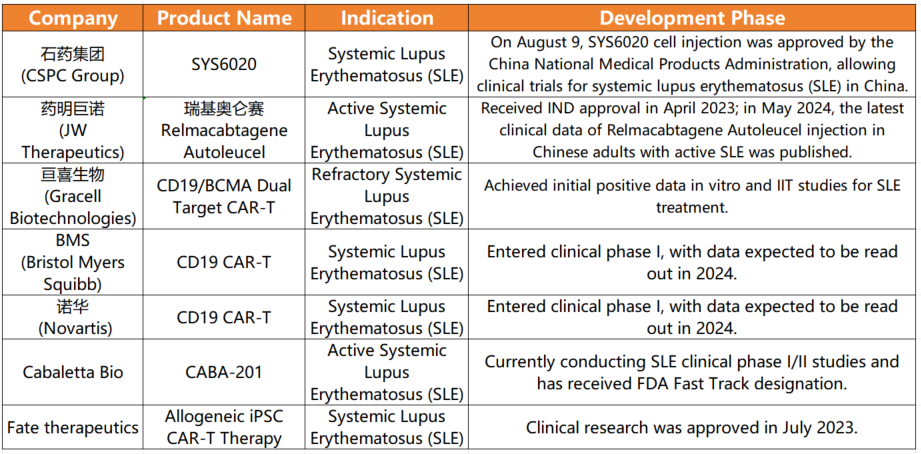
Recently, CSPC PHARMA’s CAR-T product, SYS6020, received approval for clinical trials from China’s National Medical Products Administration (NMPA). This groundbreaking development represents a significant milestone in cell therapy, especially for treating refractory active systemic lupus erythematosus (SLE). Notably, SYS6020 targets the BCMA antigen, potentially providing a novel and cost-effective treatment option for SLE patients worldwide.
**Innovative Approach and Promising Safety Profile**
SYS6020 is a pioneering mRNA-LNP-based CAR-T cell injection, marking it as the first of its kind to enter clinical trials. This innovative therapy specifically recognizes the BCMA antigen, attacking and eliminating immune cells responsible for elevated autoantibodies. This approach offers a unique, safe, and effective treatment option for SLE, distinct from traditional CAR-T therapies. Notably, SYS6020 exhibits high cell viability, a high CAR-positive rate, and reduced risks of genomic integration and cytokine release syndrome (CRS), common issues with conventional CAR-T treatments.
Preclinical studies have shown that SYS6020 effectively kills BCMA antigen-positive myeloma cells while maintaining a favorable safety profile. By employing LNP for T cell transfection, the therapy significantly reduces costs compared to using lentiviral vectors, lessening the financial burden on patients.
Lupus
**Revolutionizing Treatment for Autoimmune Diseases**
SLE, a complex chronic autoimmune disease, is characterized by the immune system attacking its own tissues. According to the “Chinese Guidelines for Diagnosis and Treatment of Lupus Nephritis,” the prevalence of SLE in China is estimated to be between 30.13 and 70.41 per 100,000 individuals, translating to 422,000 to 986,000 patients.
Traditional SLE treatments, including steroids, often come with severe side effects and long-term health risks. In contrast, biological therapies have emerged as a game-changer by modulating immune responses, reducing the immune system’s attack on the body’s tissues, and decreasing dependency on steroids. However, only three biologics—belimumab, tildrakizumab, and anifrolumab—are currently approved globally.
CAR-T therapy has shown remarkable efficacy in treating refractory SLE, providing hope in a challenging therapeutic landscape. This therapy precisely targets “rogue” B cells responsible for autoantibody production, thereby minimizing organ damage and offering a more targeted approach than traditional treatments.
**Future Prospects and Ongoing Challenges**
While CAR-T therapy presents a promising new direction for SLE treatment, its long-term efficacy and safety remain under investigation. Larger clinical trials are needed to validate its effectiveness and safety, as current studies are limited to small samples.
Additionally, the potential long-term risks of CAR-T therapy, such as increased susceptibility to infections or malignancies, require thorough exploration. Despite these uncertainties, the active involvement of multiple domestic and international cell therapy companies underscores the global commitment to advancing CAR-T therapies for autoimmune diseases.
In summary, CAR-T therapy for SLE holds immense potential, with clinical trials offering hope for a safer and more effective treatment alternative. Continued research and positive trial outcomes could establish CAR-T as a viable therapeutic option for SLE patients, revolutionizing their treatment landscape.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#CAR_Therapy #SLETreatment #AutoimmuneInnovation #MedicalBreakthrough #LupusAwareness
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

“Thank you,” Teresa said to the doctors and nurses with a smile, “Your professionalism and care make me feel incredibly reassured. I believe that with you by my side, I will conquer this illness.”
### Gratitude to the Doctors and Nurses at Jiahui International Hospital in Shanghai:
After undergoing the processes of apheresis and reinfusion, Teresa feels immense gratitude towards the doctors and nurses at Jiahui International Hospital in Shanghai. She has deeply experienced the professionalism and care of the medical staff.
Under the guidance of Dr. Vicky Lee and her team, the entire collection and infusion process became smooth and reassuring. Every doctor and nurse patiently explained each step, effectively prevented and assessed the risks that might occur after reinfusion, and constantly monitored her physical condition and emotional changes, providing meticulous care and comfort. They also offered tremendous psychological support. The warm words and determined eyes of the doctors and nurses gave Teresa strength during moments of uncertainty.
It is precisely the professionalism and compassion of these healthcare workers that filled Teresa with confidence and hope on her journey to fight the illness. She sincerely thanks all the medical staff at Jiahui Hospital. Their dedication and care have shown her the hope of recovery.
We will continue to follow up on the patient’s treatment progress and provide updates.
#CART #CARTTherapy #Hopeforpatients #FUCASO #Equecel #MultipleMyeloma #jihuiHospital #Shanghai #ChineseCart #MedicalInnovation #MedicalBreakthrough #CancerTreatment #FullyHumanCART #cancerfight #cancersurvivor #Jiahuihospital
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

“谢谢你们,”Teresa微笑着对医生和护士们说道,“你们的专业和关爱让我感到无比安心。我相信,有了你们,我会战胜这场病魔。”
感谢上海嘉会国际医院的医生护士:
在经历了单采,回输等过程后,Teresa对上海嘉会国际医院的医生和护士们心怀感激。她深深体会到了医护人员的专业和关怀。
在Dr. Vicky Lee及其团队的指导下,整个采集输注过程变得顺利和安心。每一位医生和护士都耐心解释每一个步骤,甚至对回输后会出现的风险都做了有效预防和评估,时刻关注她的身体状况和情绪变化,给予她无微不至的照顾和安慰。并且在心理上给予了莫大的支持。医生和护士的温暖话语和坚定目光,让Teresa在不安时找到了力量。
正是这些医护人员的专业和爱心,让Teresa在对抗病魔的路上充满了信心和希望。她由衷地感谢嘉会医院的全体医护人员,是他们的付出和关怀,让她看到了康复的希望。
我们将持续关注患者的治疗后续,并跟进报道。
#CART #CARTTherapy #Hopeforpatients #FUCASO #Equecel #MultipleMyeloma #jihuiHospital #Shanghai #ChineseCart #MedicalInnovation #MedicalBreakthrough #CancerTreatment #FullyHumanCART #cancerfight #cancersurvivor #Jiahuihospital
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Blood Cancer Solution Including leukemia, lymphoma, multiple myeloma, and others.
 Blood Cancer Solution
Blood Cancer Solution
 Including leukemia, lymphoma, multiple myeloma, and others.
Including leukemia, lymphoma, multiple myeloma, and others.

Blood Cancer
#leukemia #lymphoma #multiplemyeloma
#Hematologic malignancies are a group of malignant diseases originating from hematopoietic cells, often affecting the bone marrow, blood, and various organs and tissues throughout the body. Common types of hematologic malignancies include leukemia, myelodysplastic syndromes, lymphomas, multiple myeloma, and myeloproliferative neoplasms.
The causes of these diseases are complex, involving genetic mutations, immune abnormalities, radiation exposure, contact with harmful chemicals, infections, and hereditary factors. Additionally, poor lifestyle habits, high levels of stress, and environmental factors can also increase the risk of developing these conditions.
With an aging population and advancements in medical technology, the incidence of hematologic malignancies has been rising globally. In China, the incidence and mortality rates of leukemia and lymphoma are now among the top ranks of all malignancies.
However, hematologic malignancies are not incurable. In recent years, the treatment methods for these diseases have seen significant progress. From traditional combination chemotherapy and radiotherapy to hematopoietic stem cell transplantation, monoclonal antibody therapy, antibody-drug conjugates, small molecule targeted therapies, and the latest immunotherapies, treatment options have become increasingly diverse and precise.
Combination chemotherapy remains a primary treatment for many hematologic malignancies, despite its significant side effects. The efficacy of these treatments cannot be ignored. Modern chemotherapy regimens are continually being refined, including the incorporation of new cytotoxic drugs and targeted therapies, as well as the use of monoclonal antibodies. Additionally, the appropriate use of antiemetics, hematopoietic growth factors, and anti-infective agents helps to mitigate adverse effects.
Hematopoietic stem cell transplantation continues to be one of the most effective treatments for certain hematologic malignancies. The development of this treatment in China has been rapid, with 170 registered transplant centers by 2020.
Monoclonal antibodies, often referred to as “biological missiles,” have a high degree of specificity and single biological activity. They have revolutionized the treatment of hematologic malignancies. Antibody-drug conjugates (ADCs) utilize monoclonal antibodies to accurately identify tumor cell markers, guiding the delivery of chemotherapy drugs for targeted treatment.
Small molecule targeted therapies work by interfering with specific genes or proteins to inhibit tumor cell growth and proliferation. Gleevec, the first small molecule targeted therapy, increased the five-year survival rate for chronic myeloid leukemia (CML) patients from 30% to 89%, marking a breakthrough in cancer treatment. Today, there are numerous small molecule targeted drugs available for the treatment of hematologic malignancies, including BCR-ABL inhibitors, BTK inhibitors, BCL-2 inhibitors, PI3K inhibitors, and XPO1 inhibitors, with many more drugs currently in clinical trials expected to become available soon.
Immunotherapy includes immune checkpoint inhibitors (such as PD-1/L1), cancer vaccines, cellular immunotherapies (such as #CART), and nonspecific immunomodulatory treatments. #CARTtherapy, in particular, has gained widespread attention as an emerging curative treatment. This approach involves extracting a patient’s T cells, modifying them outside the body to specifically recognize and attack tumor cells, and then reinfusing the modified T cells into the patient. This therapy has been successfully applied to various hematologic malignancies, including acute lymphoblastic leukemia, lymphomas, and multiple myeloma. The first patient treated with CAR-T therapy has been disease-free for 11 years.
In recent years, China has made significant advances in the treatment of hematologic malignancies. The establishment of the “Chinese Expert Consensus on the Diagnosis and Treatment of High-Risk Multiple Myeloma” and the presentation by Professor Huang He at the 2024 #EHA conference on targeting CD7 universal CAR-T therapy for T-cell acute lymphoblastic leukemia (T-ALL) and T-cell lymphoblastic lymphoma (T-LBL) have shown remarkable efficacy and safety. Additionally, exciting new data from the 2024 American Society of Clinical Oncology (#ASCO) annual meeting highlighted the efficacy of Relma-cel in treating relapsed/refractory large B-cell lymphoma (R/R LBCL), with a four-year overall survival rate (#OS) of 66.7%. Particularly noteworthy is the research on multiple myeloma, where the BCMA-targeted CAR-T therapy has demonstrated deep and lasting responses, with a complete response (#CR) rate of 82.4% and a 12-month progression-free survival (#PFS) rate of 85.5%.
With the continuous development of new treatments and the emergence of new drugs, hematologic malignancies in China are no longer considered incurable diseases. Through standardized, individualized, and precise treatments, many patients with hematologic malignancies can achieve long-term disease-free survival, and even a cure, returning to normal work and life. As medicine continues to advance, every life will continue to shine brightly!

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#HematologicMalignancies #LeukemiaAwareness #LymphomaResearch #MultipleMyeloma #BloodCancer #CancerResearch #CAR_Therapy #StemCellTransplant #Immunotherapy #TargetedTherapy #MonoclonalAntibodies #CancerTreatment #MedicalAdvancements #CancerSurvivor #HealthcareInnovation
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Light of Hope – The Starting Point for Multiple Myeloma Patients
