Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Why Are More and More International Patients Coming to China for CAR-T Cell Therapy?
Why Are More and More International Patients Coming to China for CAR-T Cell Therapy?

CARTtherapy
#CARTtherapy #InternationalPatient #CART #Multiplemyeloma #lymphoma #leukemia
In recent years, CAR-T cell therapy, as an innovative cancer treatment, has rapidly gained widespread attention globally. China, as a leading country in the CAR-T treatment field, has attracted an increasing number of patients from Europe and Asia due to its advanced technology, high-quality medical resources, and relatively lower treatment costs. So, why are more international patients choosing to come to China for CAR-T treatment? Here are some key reasons worth noting:
### 1. **Advanced CAR-T Treatment Technology**
Over the past decade, China has made tremendous strides in medical research, particularly in the field of cancer immunotherapy. Not only has China successfully developed various CAR-T therapies, but it has also built a comprehensive treatment protocol and safety monitoring system to ensure efficient and safe treatment for patients. Additionally, China has accumulated extensive experience in managing treatment-related side effects, such as CRS (Cytokine Release Syndrome) and neurotoxicity.
Currently, more than 50% of the world’s clinical trials for CAR-T therapy are conducted in China. Of the 11 CAR-T products already on the global market, five are from China. These include products for multiple myeloma (BCMA CAR-T), diffuse large B-cell lymphoma (CD19 CAR-T), and leukemia. Compared to similar products in the U.S. for multiple myeloma, China’s CAR-T products are not only fully humanized but also boast the highest complete remission rates among all available products, reaching 82.4%. The CR data for China’s lymphoma and leukemia CAR-T products are also impressive, at 77.6% and 82.1%, respectively.
### 2. **International Certification and Clinical Trial Opportunities**
Several hospitals and research institutions in China have gained international recognition for their CAR-T therapies. Patients can choose between commercial CAR-T treatments or participate in clinical trials. For some CAR-T treatments that have not yet been approved or are hard to access in Europe or Asia, patients can receive these cutting-edge therapies in China earlier.
Just a few days ago, Chinese scholars’ research on universal CAR-T cell therapy was featured on the cover of *Nature*. Various other studies on Chinese CAR-T therapies are frequently published in major medical journals. For example, a Chinese medical team published a study on dual-target CAR-T in *JAMA Oncology*, showing a 100% stringent complete remission (sCR) rate in high-risk multiple myeloma patients. Additionally, a research team from Zhejiang, China, published clinical data in *Cell Discovery*, highlighting the breakthrough of the fourth-generation anti-CD19 CAR-T cell therapy in treating relapsed/refractory large B-cell lymphoma with exceptional anti-tumor capabilities.
### 3. **Relatively Low Treatment Costs**
Compared to the million-dollar treatment costs in Western countries, CAR-T treatment in China is relatively affordable, often costing just 1/5 to 1/7 of the price in the U.S. For instance, the cheapest CAR-T product for multiple myeloma in the U.S. costs $420,000, and when including hospitalization and other expenses, the total cost can reach around $700,000. In contrast, similar Chinese products, with equal or even better effectiveness, cost only $160,000, and hospitalization and other fees are much lower, often under $10,000. Some treatment options are available for just a few tens of thousands of dollars, and in certain cases, treatment is even completely free.
Patients from Europe and Asia can receive world-class CAR-T therapy in China at a much lower cost, providing an affordable option for those who find the high costs of treatment elsewhere prohibitive. Moreover, a range of one-stop medical services, including hospital visits, translation assistance, transportation, accommodation, and even family care, can significantly enhance the patient experience for international patients.
### 4. **World-Class Expert Teams**
China is home to a group of internationally renowned oncology and immunotherapy experts who have extensive clinical experience and academic achievements in hematological cancers and CAR-T therapy. Patients receive personalized treatment plans designed by leading experts, ensuring that each treatment is precisely tailored to their specific condition, maximizing effectiveness and minimizing risks.
For example, Professor Huang Xiaojun, Director of the Institute of Hematology at Peking University, is one of the world’s pioneers in haploidentical transplantation, having developed China’s haploidentical hematopoietic stem cell transplantation model. His innovative approach has increased the 3-year survival rate of leukemia patients receiving haploidentical transplants from around 20% to approximately 70%, earning him the Distinguished Service Award from the Center for International Blood and Marrow Transplant Research.
### 5. **Efficient and Convenient Medical Services**
China has implemented a 144-hour visa-free transit policy for citizens of 54 countries and mutual visa exemptions with 24 countries. This is a major benefit for international patients seeking treatment in China. Moreover, China’s healthcare system is known for its efficiency and convenience, especially in treating major diseases. Appointment wait times are short, and there is minimal delay in receiving treatment. A “super green channel” is also available for international patients, ensuring quick diagnosis and treatment planning. From initial consultation to online meetings with specialists, the process typically takes less than a week, with hospital admission and treatment beginning within another week.
Compared to the long waiting periods in other countries, China’s efficient medical services significantly improve treatment success rates and patient satisfaction. CAR-T therapy, in particular, is known for its high efficiency. There was an international patient who went from initial consultation to complete remission and a safe return home in less than a month—a remarkable achievement considering CAR-T is a “living” drug that must be custom-prepared using the patient’s own blood cells.
### 6. **Abundant Success Stories**
Many international patients who have received CAR-T therapy in China have achieved remarkable results, with their cancers being effectively controlled or even cured. These success stories not only boost confidence among patients worldwide but also attract more people to China for treatment. By sharing these stories, more patients learn about China’s advantages in CAR-T therapy and choose it as their treatment destination.
For instance, Ethan, a patient from Singapore, achieved complete remission just four weeks after CAR-T treatment and has since recovered very well. There is also the story of a Russian patient with high-risk, late-stage multiple myeloma who received efficient treatment in China at a much more reasonable cost and with a faster treatment process.
### 7. **Conclusion**
In conclusion, as China continues to enhance its technological capabilities in CAR-T cell therapy, more and more patients from around the world are choosing to come to China for this cutting-edge cancer treatment. With its advanced medical technology, international services, and relatively lower treatment costs, China is becoming an important destination for cancer patients seeking treatment. If you or a loved one is searching for innovative cancer treatment options, consider CAR-T therapy in China for a chance at recovery!
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can contact us for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CellTherapy #CancerTreatment #Immunotherapy #HealthcareInnovation #MedicalTourism #ChinaHealthcare #CancerSurvivor #PatientStories #MedicalAdvancements #AffordableHealthcare #PersonalizedMedicine #GlobalHealth #HealthJourney #Oncology
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

**Chinese Medical Team Releases Dual-Target CAR-T Data: 100% Complete Remission Rate in High-Risk MM Patients**
**Chinese Medical Team Releases Dual-Target CAR-T Data: 100% Complete Remission Rate in High-Risk MM Patients**

High-Risk MM
#CARTTherapy #MultipleMyeloma #BCMA #CD19 #JAMAOncology #MM #HRMM #DualTarget
Once again, a Chinese medical team is at the forefront of innovation, making significant strides in the field of CAR-T cell therapy. A recent study published in *JAMA Oncology* (B-Cell Maturation Antigen/CD19 Dual-Targeting Immunotherapy in Newly Diagnosed Multiple Myeloma) revealed remarkable results for the dual-target BCMA/CD19 CAR-T therapy in high-risk newly diagnosed multiple myeloma (NDMM) patients. In this study, all 19 participants achieved stringent complete remission (sCR) and were found to be minimal residual disease (MRD) negative, resulting in an astounding 100% remission rate.
**CAR-T Therapy: A Breakthrough in Hematologic Cancer Treatment**
Multiple myeloma (MM) is a challenging hematologic cancer, particularly for patients with high-risk features, where traditional treatments often show limited efficacy. CAR-T cell therapy is an innovative immunotherapy that reprograms a patient’s own T cells to recognize and attack cancer cells. In recent years, this therapy has shown significant success in treating leukemia, lymphoma, and relapsed/refractory MM (RRMM). The latest study further demonstrates that dual-target CAR-T therapy, focusing on BCMA and CD19, can significantly improve the prognosis for high-risk MM patients.
**The Impressive Results of China’s Dual-Target CAR-T Therapy**
The CAR-T therapy used in this study, GC012F, targets both B-cell maturation antigen (BCMA) and CD19 surface antigens, showing powerful anti-tumor effects. Every patient in the study not only achieved complete remission after treatment but also maintained a long-term MRD-negative status, meaning that traces of cancer cells were nearly undetectable post-therapy.
More importantly, the study highlighted the swift effectiveness of the Chinese GC012F therapy—patients reached their first complete remission in a median time of just 84 days, with MRD-negative status achieved in as little as 28 days. This rapid anti-tumor response provides a crucial treatment window for patients, significantly improving their prognosis.
**Safety and Future Prospects**
In addition to its impressive efficacy, GC012F demonstrated favorable safety. Only 27% of patients experienced mild to moderate cytokine release syndrome (CRS), and no cases of immune effector cell-associated neurotoxicity syndrome (ICANS) were observed. This is in stark contrast to previous studies in relapsed/refractory patients, where CRS rates were higher, highlighting the safety advantage of this therapy in newly diagnosed patients.
Though the study sample size was small, these findings bring new hope for high-risk multiple myeloma patients. As larger clinical trials are conducted and combination therapies are explored, this therapy has the potential to offer better survival outcomes for more patients.
**Conclusion**
China’s CAR-T cell therapy research continues to lead the way in hematologic cancer treatment. The success of the BCMA/CD19 dual-target CAR-T therapy not only demonstrates its strong efficacy in high-risk multiple myeloma but also provides a new direction for treating hematologic cancers globally. As research deepens, we anticipate that this groundbreaking therapy will bring new life-saving opportunities to more patients.
This breakthrough in Chinese CAR-T therapy represents not only scientific progress but also underscores China’s pivotal role in the global fight against cancer.
To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CancerTreatment #Immunotherapy #MedicalInnovation #CancerResearch #ChinaHealthcare #HematologicCancer #CancerBreakthrough
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese CAR-T Therapy: A New Frontier in Treating Systemic Lupus Erythematosus
Chinese CAR-T Therapy: A New Frontier in Treating Systemic Lupus Erythematosus

Systemic Lupus Erythematosus
Recently, CSPC PHARMA’s CAR-T product, SYS6020, received approval for clinical trials from China’s National Medical Products Administration (NMPA). This groundbreaking development represents a significant milestone in cell therapy, especially for treating refractory active systemic lupus erythematosus (SLE). Notably, SYS6020 targets the BCMA antigen, potentially providing a novel and cost-effective treatment option for SLE patients worldwide.
**Innovative Approach and Promising Safety Profile**
SYS6020 is a pioneering mRNA-LNP-based CAR-T cell injection, marking it as the first of its kind to enter clinical trials. This innovative therapy specifically recognizes the BCMA antigen, attacking and eliminating immune cells responsible for elevated autoantibodies. This approach offers a unique, safe, and effective treatment option for SLE, distinct from traditional CAR-T therapies. Notably, SYS6020 exhibits high cell viability, a high CAR-positive rate, and reduced risks of genomic integration and cytokine release syndrome (CRS), common issues with conventional CAR-T treatments.
Preclinical studies have shown that SYS6020 effectively kills BCMA antigen-positive myeloma cells while maintaining a favorable safety profile. By employing LNP for T cell transfection, the therapy significantly reduces costs compared to using lentiviral vectors, lessening the financial burden on patients.
Lupus
**Revolutionizing Treatment for Autoimmune Diseases**
SLE, a complex chronic autoimmune disease, is characterized by the immune system attacking its own tissues. According to the “Chinese Guidelines for Diagnosis and Treatment of Lupus Nephritis,” the prevalence of SLE in China is estimated to be between 30.13 and 70.41 per 100,000 individuals, translating to 422,000 to 986,000 patients.
Traditional SLE treatments, including steroids, often come with severe side effects and long-term health risks. In contrast, biological therapies have emerged as a game-changer by modulating immune responses, reducing the immune system’s attack on the body’s tissues, and decreasing dependency on steroids. However, only three biologics—belimumab, tildrakizumab, and anifrolumab—are currently approved globally.
CAR-T therapy has shown remarkable efficacy in treating refractory SLE, providing hope in a challenging therapeutic landscape. This therapy precisely targets “rogue” B cells responsible for autoantibody production, thereby minimizing organ damage and offering a more targeted approach than traditional treatments.
**Future Prospects and Ongoing Challenges**
While CAR-T therapy presents a promising new direction for SLE treatment, its long-term efficacy and safety remain under investigation. Larger clinical trials are needed to validate its effectiveness and safety, as current studies are limited to small samples.
Additionally, the potential long-term risks of CAR-T therapy, such as increased susceptibility to infections or malignancies, require thorough exploration. Despite these uncertainties, the active involvement of multiple domestic and international cell therapy companies underscores the global commitment to advancing CAR-T therapies for autoimmune diseases.
In summary, CAR-T therapy for SLE holds immense potential, with clinical trials offering hope for a safer and more effective treatment alternative. Continued research and positive trial outcomes could establish CAR-T as a viable therapeutic option for SLE patients, revolutionizing their treatment landscape.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#CAR_Therapy #SLETreatment #AutoimmuneInnovation #MedicalBreakthrough #LupusAwareness
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

“Thank you,” Teresa said to the doctors and nurses with a smile, “Your professionalism and care make me feel incredibly reassured. I believe that with you by my side, I will conquer this illness.”
### Gratitude to the Doctors and Nurses at Jiahui International Hospital in Shanghai:
After undergoing the processes of apheresis and reinfusion, Teresa feels immense gratitude towards the doctors and nurses at Jiahui International Hospital in Shanghai. She has deeply experienced the professionalism and care of the medical staff.
Under the guidance of Dr. Vicky Lee and her team, the entire collection and infusion process became smooth and reassuring. Every doctor and nurse patiently explained each step, effectively prevented and assessed the risks that might occur after reinfusion, and constantly monitored her physical condition and emotional changes, providing meticulous care and comfort. They also offered tremendous psychological support. The warm words and determined eyes of the doctors and nurses gave Teresa strength during moments of uncertainty.
It is precisely the professionalism and compassion of these healthcare workers that filled Teresa with confidence and hope on her journey to fight the illness. She sincerely thanks all the medical staff at Jiahui Hospital. Their dedication and care have shown her the hope of recovery.
We will continue to follow up on the patient’s treatment progress and provide updates.
#CART #CARTTherapy #Hopeforpatients #FUCASO #Equecel #MultipleMyeloma #jihuiHospital #Shanghai #ChineseCart #MedicalInnovation #MedicalBreakthrough #CancerTreatment #FullyHumanCART #cancerfight #cancersurvivor #Jiahuihospital
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

“谢谢你们,”Teresa微笑着对医生和护士们说道,“你们的专业和关爱让我感到无比安心。我相信,有了你们,我会战胜这场病魔。”
感谢上海嘉会国际医院的医生护士:
在经历了单采,回输等过程后,Teresa对上海嘉会国际医院的医生和护士们心怀感激。她深深体会到了医护人员的专业和关怀。
在Dr. Vicky Lee及其团队的指导下,整个采集输注过程变得顺利和安心。每一位医生和护士都耐心解释每一个步骤,甚至对回输后会出现的风险都做了有效预防和评估,时刻关注她的身体状况和情绪变化,给予她无微不至的照顾和安慰。并且在心理上给予了莫大的支持。医生和护士的温暖话语和坚定目光,让Teresa在不安时找到了力量。
正是这些医护人员的专业和爱心,让Teresa在对抗病魔的路上充满了信心和希望。她由衷地感谢嘉会医院的全体医护人员,是他们的付出和关怀,让她看到了康复的希望。
我们将持续关注患者的治疗后续,并跟进报道。
#CART #CARTTherapy #Hopeforpatients #FUCASO #Equecel #MultipleMyeloma #jihuiHospital #Shanghai #ChineseCart #MedicalInnovation #MedicalBreakthrough #CancerTreatment #FullyHumanCART #cancerfight #cancersurvivor #Jiahuihospital
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

希望之光-多发性骨髓瘤患者的起点
希望之光-多发性骨髓瘤患者的起点
Teresa女士坐在单采室的舒适座位上,注视着护士轻柔地在她手臂上穿刺,抽取宝贵的白细胞样本。这些白细胞将成为CAR-T治疗的核心材料,经过工程化后,将再次注入她体内,成为对抗癌症的力量源泉。
手术室内,机器轻声运转,精密地分离出Teresa身体中的T细胞。这些细胞,此前在她的身体内忠实执行着保护机体的职责,如今将被重新教育,成为一支针对癌细胞的精确打击队伍。Teresa闭上眼睛,心中默默祈祷这次单采能为她的身体注入战斗的力量,将病魔赶出她的生命。
单采过程持续了几个小时,医护人员细心地监控着每一个步骤,确保采集到足够数量和质量的细胞。这些细胞的提取不仅是技术上的挑战,更是对医疗团队专业能力的严格考验。
完成单采后,Teresa感到轻松了一些。她知道,这只是漫长治疗过程中的第一步,但也是希望之光的重要起点。在单采室内,她感受到了医护团队的温暖和专业,这让她对未来的治疗充满信心和希望。
回到病房,Teresa在床上轻轻闭上了眼睛。她思考着接下来的日子将如何展开,希望这次单采能为她带来战胜疾病的力量,让她重获新生。
单采,作为治疗之旅的第一步,铸就了Teresa勇敢抗癌的新篇章,也为她未来的康复之路点燃了希望的火光。
#希望之路 #ThePathOfHope #与癌症抗争 #FightingCancer #CART治疗之旅 #JourneyOfCARTTherapy #携手同济 #TogetherWithTongji #全人源CART #FullyHumanCART #FUCASO希望 #HopeWithFUCASO #EquecelApproval #健康重生 #HealthReborn #共同祈愿 #UnitedInPrayer #MultipleMyeloma
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China’s Breakthrough in CAR-T Therapy for CNS Lymphoma
### China’s Breakthrough in CAR-T Therapy for CNS Lymphoma

Lymphoma
In a pioneering effort to improve outcomes for patients with refractory/relapsed central nervous system lymphoma (CNSL), a multi-center retrospective study led by Professor Jianqing Mi from Ruijin Hospital, Shanghai Jiaotong University School of Medicine, has showcased the impressive real-world effectiveness of Relmacabtagene Autoleucel (Relma-cel). This study, encompassing data from 12 centers across China, represents the largest sample size for a real-world study on commercial CAR-T therapy in treating CNSL, and its results have been recently published in the *Journal for ImmunoTherapy of Cancer*.
### Study Overview and Patient Demographics
The study included 22 patients aged 18 and above, all diagnosed with CD19+ refractory or relapsed CNSL. These patients had previously undergone various systemic treatments, including CD20 monoclonal antibody immunochemotherapy and high-dose methotrexate-based therapies. Of the participants, 12 had primary CNSL and 10 had secondary CNSL. The median age was 56, with 45.5% being over 60 years old. The study focused on a high-risk group, with many having a Karnofsky Performance Status (KPS) score of ≤60, multiple prior treatment lines, and/or high-risk genetic profiles such as double-hit lymphoma (DHL).
### Treatment and Response
Patients received Relma-cel with a median interval of 32 days between apheresis and infusion. Thirteen patients received a single CAR-T cell infusion, while nine underwent autologous stem cell transplantation (ASCT) in combination with CAR-T infusion. Notably, 20 patients received bridging therapy to control disease before CAR-T infusion. The overall response rate (ORR) was 90.9%, with a complete response (CR) rate of 68.2%. Impressively, all patients achieved CNS response, with 72.7% achieving CNS CR. The median time to response was one month.
### Follow-Up and Survival Outcomes
The median follow-up period was 316 days. Among the 16 patients who achieved CNS response, 81.3% remained alive and in remission, with half maintaining CNS CR for over a year. The study reported a one-year progression-free survival (PFS) rate of 64.4%, duration of response (DOR) rate of 71.5%, and overall survival (OS) rate of 79.2%. Key predictors of better outcomes included achieving CR before infusion and having non-progressive disease at the time of infusion.
### Safety and Tolerability
The safety profile of Relma-cel was acceptable. Cytokine release syndrome (CRS) occurred in 72.7% of patients, primarily grade 1 or 2, with only one case of grade 3. Immune effector cell-associated neurotoxicity syndrome (ICANS) was reported in 36.4% of patients, mostly grade 1 or 2. There were no CAR-T therapy-related deaths, although five patients (22.7%) died, with three deaths due to disease progression and two from non-relapse causes (COVID-19).
### CAR-T Cell Dynamics and Combined Therapy
The study also explored the pharmacokinetics of CAR-T cells. Relma-cel showed significant expansion in the peripheral blood within the first 28 days post-infusion, with CAR-T cells detected in the cerebrospinal fluid of all evaluable patients. Interestingly, patients receiving additional immunotherapies like BTK inhibitors or PD-1 inhibitors exhibited CAR-T cell re-expansion, suggesting potential synergistic effects.
### Conclusion and Future Directions
This landmark study underscores the clinical efficacy and manageable safety profile of Relma-cel for treating CNSL in a real-world setting. It highlights the potential benefits of combining CAR-T therapy with other immunotherapies, offering a promising strategy for enhancing CAR-T cell persistence and effectiveness. These findings pave the way for future research, suggesting the need for larger, randomized studies to further validate these results and explore the role of CAR-T therapy as a consolidation treatment for high-risk CNSL patients. As China continues to advance in medical research and technology, studies like this are crucial in providing valuable insights and improving global healthcare standards.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#CARTTherapy #CNSLymphoma #CancerResearch #MedicalBreakthrough #ChinaHealthcare #Immunotherapy #RelmaCel #PatientOutcomes #InnovativeTreatment #ClinicalStudy
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Interpretation of Hot Topics Conerning Relapsed/Refractory Mutiple Myeloma Patients
 Interpretation of Hot Topics Conerning Relapsed/Refractory Mutiple Myeloma Patients:
Interpretation of Hot Topics Conerning Relapsed/Refractory Mutiple Myeloma Patients:
What other treatment options are available for patients with relapsed Multiple Myeloma?
How effective is the new drug treatment for the first relapse of Multiple Myeloma?
What treatment options are available for initial relapse of multiple myeloma?
What are the treatment goals for Relapsed/Refractory Multiple Myeloma (RRMM)?
What treatment strategy should be adopted for RRMM in order to achieve maximum relief?
For Relapsed/Refractory Multiple Myeloma, what are the Grades of Response?
Expert Introduction
👩🔬Zhuang Junling
Associate Chief Physician Department of Hematology
Peking Union Medical College Hospital
Published over 50 papers in journals such as Blood, Leukemia Research, and others.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#CART #Mutiplemyeloma #CARTtherapy #myeloma #leukemia #drug #RRMM #cancer #cancersurvivor #cancerpatient #bloodcancer #tumor #cartcell #cartcelltherapy #cancertreatment #advancedmedicineinchina #hematology
The Emergence of Fifth Generation CAR-T: A Boon for Late-Stage Cancer Patients or a Major Breakthrough in Solid Tumor Treatment?
The fifth-generation CAR-T is designed as a universal type of CAR-T. Is this risk-free CAR-T capable of achieving significant breakthroughs in solid tumor treatment, or is it effectively reducing costs to enable scalable production and treatment?
After nearly three decades of development, CAR (Chimeric Antigen Receptor) technology has undergone continuous innovation. Currently, CAR has evolved to its fifth generation. Its aim is to enhance the safety of treatments by reducing toxicity and non-specific antigen recognition. This is achieved by stimulating proliferation, activation, and the generation of memory phenotypes within CAR-T cells to improve efficiency and provide immune regulation for the optimal function of CAR-T cells.
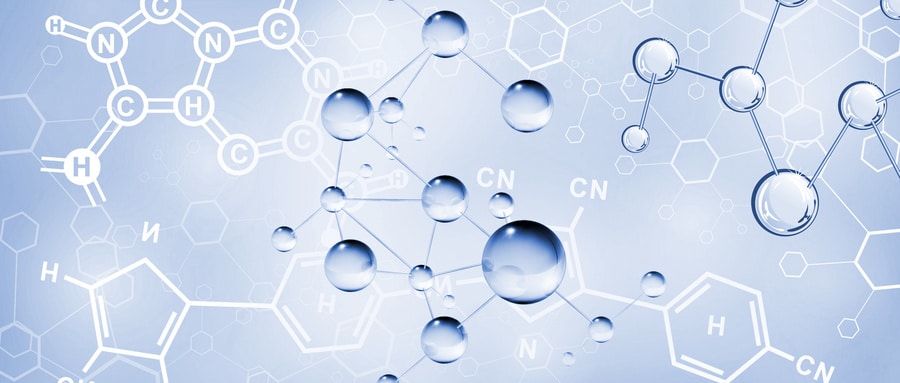
Generation CAR-T
The Evolution of Different Generations of CAR-T
First Generation CAR:
The first-generation CAR comprises an extracellular single-chain variable fragment (scFv) as the antigen recognition binding domain and an intracellular CD3ζ as the cellular activation signaling domain. Despite initiating cytotoxic anti-tumor responses within transplanted T cells, first-generation CAR-T cells exhibit lower levels of cytotoxicity and proliferation due to the CAR structure lacking co-stimulatory domains, which results in inadequate interleukin (IL)-2 production.
Second Generation CAR:
Building upon the CD3ζ signal transduction domain, the second-generation CAR includes an additional co-stimulatory signaling domain that activates T cells, significantly enhancing T cell proliferation and survival. For instance, CD28 can deliver robust activation signals, enabling T cells to achieve high levels of cytotoxic activity in a shorter duration, while 4-1BB provides prolonged activation signals, sustaining T cell-mediated killing of tumor cells. However, limitations arise in second-generation CAR-T cells utilizing retroviruses as viral vectors, restricting the length of transgene fragments they can carry. As a result, it becomes necessary to choose between incorporating CD28 and 4-1BB into T lymphocytes.
Third Generation CAR:
Third-generation CAR-T cells utilize larger DNA-carrying lentiviruses as viral vectors, allowing simultaneous incorporation of DNA fragments for both CD28 and 4-1BB into T cells. Consequently, the third-generation CAR structure encompasses two co-stimulatory domains, theoretically addressing the need for higher activation intensity and sustained survival of CAR-T cells. However, the safety concerns associated with prolonged and high-level persistence of CAR-T cells, including potential attacks on the host’s immune system, remain unresolved despite these advancements.
Fourth Generation CAR:
The design concept behind the fourth-generation CAR revolves around the precise treatment of cancerous diseases. For instance, solid tumors generate a microenvironment (TME) during their chronic progression, preventing CAR-T cells from penetrating the tumor interior. As a result, CAR-T therapy demonstrates limited efficacy in treating solid tumors. TRUCK CAR-T involves incorporating cytokines (such as IL-12) or chemokines into the CAR structure. This facilitates increased infiltration of T cells into tumor tissues while recruiting other immune cells within the body to eliminate tumor cells. In some studies, a suicide gene or certain drug-sensitive genes are attached to the CAR structure to ensure the clearance of CAR-T cells from the body post-treatment, preventing inadvertent harm to normal cells and enhancing the safety and controllability of CAR-T therapy.
Fifth Generation CAR:
The fifth-generation CAR-T, known as universal CAR-T, achieves T-cell receptor α (TCR-α) and β (TCR-β) chain deletion by knocking out the TRAC gene. This implies the removal of the T-cell receptor (TCR) from the surface of T cells, thereby avoiding the occurrence of graft-versus-host disease (GVHD) in transplantation reactions.
Since the FDA’s approval of the CD19 CAR-T product, Novartis’s Kymriah, in 2017, CAR-T cell therapy has entered a stage of rapid development. However, the currently approved and marketed products are all second-generation CAR-T therapies. There is still a long way to go for CAR-T to become widespread in the market.
Safety concerns constitute the primary challenge for CAR-T, such as off-target effects, cytokine release syndrome (CRS), and neurotoxicity (NTX). Currently available CAR-T products primarily focus on treating hematologic malignancies, with no major breakthroughs achieved yet in treating solid tumors.
In 2021, China’s NMPA approved three CAR-T products for marketing: FOSUNKITE’s Axicabtagene Ciloleucel injection, JW Therapeutics’s Relmacabtagene Autoleucel Injection, and the recently approved JUVENTAS’s Inaticabtagene Autoleucel Injection, all targeting CD19. Additionally, earlier this year, IASO Bio obtained approval for Equecabtagene Autoleucel Injection, targeting BCMA. While CAR-T targeting CD19 has shown effectiveness, its scope remains limited to B-cell-related hematologic malignancies. BCMA-targeted CAR-T is restricted to treating multiple myeloma. To address solid tumor treatment, the development of more specific and potent targets is necessary.
Among the recently released domestically developed JUVENTAS’s Inaticabtagene Autoleucel Injection, its competitive advantage lies in its price, which has decreased to below one million RMB(Approximately $140,000 US).
With the continuous advancement of molecular biology technologies, more breakthroughs are expected in CAR molecule design. This progression anticipates the development of safer and more efficient universal CAR-T therapies in the future, benefiting a broader spectrum of cancer patients.
“If you’d like to inquire about the latest cancer-fighting technologies and treatments, you can contact us.”
whatsapp:+8613717959070
#CARTCellTherapy #CancerTreatment #ScienceInnovation #GeneticMedicine #TumorTreatment #HealthcareTech #MedicalScience #CancerAwareness #PatientCare #FutureOfMedicine
