Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

**Patient Story | Chinese CAR-T Therapy: An Inspiring Journey of Changing Fate for Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma (R/R DLBCL)**
**Patient Story | Chinese CAR-T Therapy: An Inspiring Journey of Changing Fate for Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma (R/R DLBCL)**

DLBCL
#CAR-T #DLBCL #Lymphoma #CARTtherapy #RRDLBCL #Patientstory #CD19
In the field of cancer treatment, China has made remarkable strides in CAR-T cell therapy, especially in the fight against relapsed or refractory diffuse large B-cell lymphoma (R/R DLBCL). Today, we will share the story of a patient whose fate was transformed by this revolutionary treatment at a leading hospital in China.
**The Patient’s Struggle and the Introduction of CAR-T Therapy**
Mr. Wang, a 45-year-old patient from China, was diagnosed with R/R DLBCL. After undergoing the standard first-line R-CHOP chemotherapy, his cancer unfortunately relapsed. He then tried an alternative chemotherapy regimen, but the disease continued to progress. The relentless development of the lymphoma took a severe toll on his physical and mental health, and he and his family were urgently seeking a more effective solution.
At the point of near despair, Mr. Wang reached out to the Advanced Medicine In China team and received a potential solution at a renowned cancer center within the team. The experts introduced him to the possibility of CAR-T cell therapy. This cutting-edge treatment, gaining increasing attention within China’s medical field, offered a glimmer of hope.
**The Treatment Process and the Expertise of the Medical Team**
Before beginning CAR-T therapy, Mr. Wang underwent a comprehensive series of evaluations and preparations. An experienced medical team of oncologists, hematologists, and immunologists meticulously planned every step of the treatment. They explained the procedure in detail to Mr. Wang and his family, addressed their concerns, and ensured they fully understood the process.
The treatment began with the collection of Mr. Wang’s own T cells. These cells were then carefully modified in an advanced laboratory to express a chimeric antigen receptor (CAR) capable of specifically recognizing the CD19 antigen on lymphoma cell surfaces. Once the CAR-T cells were ready, they were infused back into Mr. Wang. Throughout the treatment, the medical team closely monitored his condition around the clock, prepared to manage any potential side effects, as these are a known risk of CAR-T therapy.
**Overcoming Challenges and Achieving Success**
In the days following the CAR-T cell infusion, Mr. Wang experienced some expected side effects, including fever and mild fatigue. The medical team, well-versed in managing CAR-T-related toxicities, promptly implemented appropriate medication and supportive care measures. They adjusted the treatment plan as needed to ensure Mr. Wang’s comfort and safety while maximizing the efficacy of the CAR-T cells.
Miraculously, within just one month of the infusion, Mr. Wang’s symptoms began to significantly improve. His tumor markers started to decrease, and follow-up scans showed a noticeable reduction in the size of lymphoma lesions. By the third month, he achieved partial remission (PR), and to everyone’s delight, by the sixth month, he reached complete remission (CR). This was not only a victory for Mr. Wang but also a testament to the power of Chinese CAR-T therapy and the expertise of the medical team. The hospital’s advanced facilities, combined with the doctors’ in-depth knowledge and experience, enabled them to navigate the complexities of CAR-T treatment and guide Mr. Wang toward recovery.
**Ripple Effects and Future Hope**
Mr. Wang’s story is just one of many success cases within China’s CAR-T treatment program. His experience brings hope not only to patients within China but also to countless others around the world with R/R DLBCL. It showcases the potential of China’s CAR-T cell therapy to offer new life for those who have exhausted traditional treatment options.
As China continues to invest in research and development in the field of CAR-T therapy, and as more hospitals and medical teams gain proficiency in its application, the future looks increasingly hopeful. Through ongoing efforts to optimize treatment protocols, manage side effects more effectively, and expand the accessibility of this life-saving therapy, Chinese CAR-T therapy will have a growing impact on the global fight against cancer.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CancerTreatment #RRLymphoma #ChineseMedicalAdvances #Immunotherapy #PatientSuccess #Oncology #HopeForCancer #MedicalBreakthrough #CancerResearch #China
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Breakthrough in China’s CAR-T Therapy for Treating Relapsed B-ALL: Donor-Derived CD19 CAR-T Shows Long-Term Survival Advantage
**Breakthrough in China’s CAR-T Therapy for Treating Relapsed B-ALL: Donor-Derived CD19 CAR-T Shows Long-Term Survival Advantage**

B-ALL
#CAR_TTherapy #B_ALL #DonorCAR_T #CAR_T #ALL #ChinaCART #CD19 #alloHSCT
In recent years, China has made significant strides in CAR-T cell therapy, particularly in treating B-cell acute lymphoblastic leukemia (B-ALL). Donor-derived CAR-T therapy has shown promising efficacy for B-ALL patients who relapse after allogeneic hematopoietic stem cell transplantation (allo-HSCT), bringing new hope for long-term survival.
A study jointly published by the Chinese Academy of Medical Sciences and Chinese medical teams in the *Journal of Hematology & Oncology*, titled “Long-term survival with donor CD19 CAR-T cell treatment for relapsed patients after allogeneic hematopoietic stem cell transplantation,” indicates that patients treated with donor CD19 CAR-T cells achieved complete remission (CR) without requiring a second transplant, with a significant increase in long-term survival rates. This study followed 32 B-ALL patients who relapsed post-allo-HSCT. The median patient age was 24, and after receiving donor CD19 CAR-T therapy, they achieved complete remission or partial recovery in peripheral blood (CRi), with a median follow-up of 42 months.
Results showed that patients treated with donor CD19 CAR-T had a 2-year overall survival (OS) rate of 56.25% and an event-free survival (EFS) rate of 50.0%. The 5-year OS and EFS reached 53.13% and 46.88%, respectively, with no new long-term adverse events. These findings suggest that donor CAR-T cells are not only effective but also have long-term safety advantages over second transplantation or traditional donor lymphocyte infusion, offering a more promising treatment option for relapsed B-ALL patients.
While these results are encouraging, further development of early detection methods is needed to identify or prevent relapse at an earlier stage. Early relapse with donor CAR-T, especially within the first six months post-treatment, remains a challenge, requiring additional multi-center, prospective studies for validation. Overall, this research provides robust clinical data supporting donor CAR-T therapy for relapsed B-ALL patients post-transplant, potentially establishing a benchmark for CAR-T therapies in China on a global scale.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#ChinaMedicalBreakthrough #CancerResearch #Hematology #Oncology #LongTermSurvival #StemCellTransplantation #RelapsedLeukemia #ChineseMedicalResearch
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Significant Progress in CAR-T Therapy in China: Long-Term Effects of Combined CD19 and CD22 Treatment for Acute B-Lymphoblastic Leukemia
### Significant Progress in CAR-T Therapy in China: Long-Term Effects of Combined CD19 and CD22 Treatment for Acute B-Lymphoblastic Leukemia

Leukemia
#ALL #CAR-Ttherapy #Leukemia #CancerResearch #B_ALL #LeukemiaTreatment
Recently, a Chinese medical team published a notable study titled “Five-year outcome of CD19 combined with CD22 CAR-T cell therapy in B-ALL patients relapsed after allo-transplantation.” The research highlights the long-term efficacy of combined CD19 and CD22 CAR-T cell therapy in patients with relapsed acute B-lymphoblastic leukemia (B-ALL) after allogeneic hematopoietic stem cell transplantation (allo-HCT). This breakthrough has not only brought new hope to B-ALL patients but also attracted significant global attention.
**Background and Significance**
Acute B-lymphoblastic leukemia is a hematologic malignancy with poor prognosis, especially for patients who experience relapse after allo-HCT, where survival rates are significantly reduced. CAR-T cell therapy in China has shown increasingly positive results in treating B-ALL, particularly in targeting the CD19 antigen. However, the effects of targeting the CD22 antigen and its potential in combination with CD19 therapy are still under deeper investigation.
**Study Design and Methodology**
Based on a previous phase I clinical trial, this study involved a follow-up of 27 patients who had received CD19 CAR-T treatment. To comprehensively assess the treatment’s efficacy, the study also included three additional patients who experienced relapse with minimal residual disease (MRD) in the bone marrow. Although these patients did not meet the initial trial’s criteria, they received combined CD19 and CD22 CAR-T cell therapy under the same protocol. The CAR-T cells used in this study were second-generation designs created via lentiviral vector transfection.
**Study Results**
After a 5-year follow-up, the Chinese research team found that combined CD19 and CD22 therapy significantly improved patients’ long-term survival rates. Among the 30 patients who completed the combined therapy, the median follow-up time was 64.4 months. After two complete treatment cycles, patients maintained sustained remission. Survival analysis showed that the 3-year and 5-year overall survival rates reached 79% and 75%, respectively, with event-free survival rates of 54% and 50%. These results indicate that combined CD19 and CD22 CAR-T cell therapy offers substantial long-term efficacy for relapsed B-ALL patients.
**Conclusions and Future Outlook**
This study not only validates the potential of CAR-T cell therapy in hematologic malignancies but also provides new therapeutic insights for clinical practice. The combination of CD19 and CD22 holds promise for offering a more effective treatment option for B-ALL patients who relapse after allo-HCT, significantly improving their long-term survival rates.
As research in CAR-T cell therapy deepens, we may see more targeted approaches and optimized treatment protocols emerge. China’s active exploration and innovation in this field bring renewed hope to hematologic cancer patients worldwide, with the expectation that CAR-T therapy will further improve survival rates and quality of life for B-ALL patients in the near future.
To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of Advanced Medicine in China for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#Immunotherapy #CD19CD22Combo #StemCellTransplant #ChinaMedicalResearch #Hematology #CancerBreakthrough #LongTermSurvival #BloodCancer
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

**Chinese Medical Team Releases Dual-Target CAR-T Data: 100% Complete Remission Rate in High-Risk MM Patients**
**Chinese Medical Team Releases Dual-Target CAR-T Data: 100% Complete Remission Rate in High-Risk MM Patients**

High-Risk MM
#CARTTherapy #MultipleMyeloma #BCMA #CD19 #JAMAOncology #MM #HRMM #DualTarget
Once again, a Chinese medical team is at the forefront of innovation, making significant strides in the field of CAR-T cell therapy. A recent study published in *JAMA Oncology* (B-Cell Maturation Antigen/CD19 Dual-Targeting Immunotherapy in Newly Diagnosed Multiple Myeloma) revealed remarkable results for the dual-target BCMA/CD19 CAR-T therapy in high-risk newly diagnosed multiple myeloma (NDMM) patients. In this study, all 19 participants achieved stringent complete remission (sCR) and were found to be minimal residual disease (MRD) negative, resulting in an astounding 100% remission rate.
**CAR-T Therapy: A Breakthrough in Hematologic Cancer Treatment**
Multiple myeloma (MM) is a challenging hematologic cancer, particularly for patients with high-risk features, where traditional treatments often show limited efficacy. CAR-T cell therapy is an innovative immunotherapy that reprograms a patient’s own T cells to recognize and attack cancer cells. In recent years, this therapy has shown significant success in treating leukemia, lymphoma, and relapsed/refractory MM (RRMM). The latest study further demonstrates that dual-target CAR-T therapy, focusing on BCMA and CD19, can significantly improve the prognosis for high-risk MM patients.
**The Impressive Results of China’s Dual-Target CAR-T Therapy**
The CAR-T therapy used in this study, GC012F, targets both B-cell maturation antigen (BCMA) and CD19 surface antigens, showing powerful anti-tumor effects. Every patient in the study not only achieved complete remission after treatment but also maintained a long-term MRD-negative status, meaning that traces of cancer cells were nearly undetectable post-therapy.
More importantly, the study highlighted the swift effectiveness of the Chinese GC012F therapy—patients reached their first complete remission in a median time of just 84 days, with MRD-negative status achieved in as little as 28 days. This rapid anti-tumor response provides a crucial treatment window for patients, significantly improving their prognosis.
**Safety and Future Prospects**
In addition to its impressive efficacy, GC012F demonstrated favorable safety. Only 27% of patients experienced mild to moderate cytokine release syndrome (CRS), and no cases of immune effector cell-associated neurotoxicity syndrome (ICANS) were observed. This is in stark contrast to previous studies in relapsed/refractory patients, where CRS rates were higher, highlighting the safety advantage of this therapy in newly diagnosed patients.
Though the study sample size was small, these findings bring new hope for high-risk multiple myeloma patients. As larger clinical trials are conducted and combination therapies are explored, this therapy has the potential to offer better survival outcomes for more patients.
**Conclusion**
China’s CAR-T cell therapy research continues to lead the way in hematologic cancer treatment. The success of the BCMA/CD19 dual-target CAR-T therapy not only demonstrates its strong efficacy in high-risk multiple myeloma but also provides a new direction for treating hematologic cancers globally. As research deepens, we anticipate that this groundbreaking therapy will bring new life-saving opportunities to more patients.
This breakthrough in Chinese CAR-T therapy represents not only scientific progress but also underscores China’s pivotal role in the global fight against cancer.
To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CancerTreatment #Immunotherapy #MedicalInnovation #CancerResearch #ChinaHealthcare #HematologicCancer #CancerBreakthrough
Global First: Chinese CAR-T Therapy Simultaneously Cures Tumor and Lupus Erythematosus

The Emergence of Fifth Generation CAR-T: A Boon for Late-Stage Cancer Patients or a Major Breakthrough in Solid Tumor Treatment?
The fifth-generation CAR-T is designed as a universal type of CAR-T. Is this risk-free CAR-T capable of achieving significant breakthroughs in solid tumor treatment, or is it effectively reducing costs to enable scalable production and treatment?
After nearly three decades of development, CAR (Chimeric Antigen Receptor) technology has undergone continuous innovation. Currently, CAR has evolved to its fifth generation. Its aim is to enhance the safety of treatments by reducing toxicity and non-specific antigen recognition. This is achieved by stimulating proliferation, activation, and the generation of memory phenotypes within CAR-T cells to improve efficiency and provide immune regulation for the optimal function of CAR-T cells.
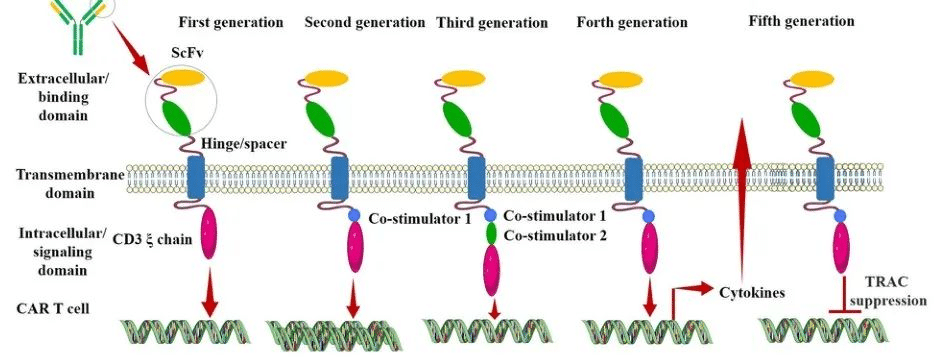
Generation CAR-T
The Evolution of Different Generations of CAR-T
First Generation CAR:
The first-generation CAR comprises an extracellular single-chain variable fragment (scFv) as the antigen recognition binding domain and an intracellular CD3ζ as the cellular activation signaling domain. Despite initiating cytotoxic anti-tumor responses within transplanted T cells, first-generation CAR-T cells exhibit lower levels of cytotoxicity and proliferation due to the CAR structure lacking co-stimulatory domains, which results in inadequate interleukin (IL)-2 production.
Second Generation CAR:
Building upon the CD3ζ signal transduction domain, the second-generation CAR includes an additional co-stimulatory signaling domain that activates T cells, significantly enhancing T cell proliferation and survival. For instance, CD28 can deliver robust activation signals, enabling T cells to achieve high levels of cytotoxic activity in a shorter duration, while 4-1BB provides prolonged activation signals, sustaining T cell-mediated killing of tumor cells. However, limitations arise in second-generation CAR-T cells utilizing retroviruses as viral vectors, restricting the length of transgene fragments they can carry. As a result, it becomes necessary to choose between incorporating CD28 and 4-1BB into T lymphocytes.
Third Generation CAR:
Third-generation CAR-T cells utilize larger DNA-carrying lentiviruses as viral vectors, allowing simultaneous incorporation of DNA fragments for both CD28 and 4-1BB into T cells. Consequently, the third-generation CAR structure encompasses two co-stimulatory domains, theoretically addressing the need for higher activation intensity and sustained survival of CAR-T cells. However, the safety concerns associated with prolonged and high-level persistence of CAR-T cells, including potential attacks on the host’s immune system, remain unresolved despite these advancements.
Fourth Generation CAR:
The design concept behind the fourth-generation CAR revolves around the precise treatment of cancerous diseases. For instance, solid tumors generate a microenvironment (TME) during their chronic progression, preventing CAR-T cells from penetrating the tumor interior. As a result, CAR-T therapy demonstrates limited efficacy in treating solid tumors. TRUCK CAR-T involves incorporating cytokines (such as IL-12) or chemokines into the CAR structure. This facilitates increased infiltration of T cells into tumor tissues while recruiting other immune cells within the body to eliminate tumor cells. In some studies, a suicide gene or certain drug-sensitive genes are attached to the CAR structure to ensure the clearance of CAR-T cells from the body post-treatment, preventing inadvertent harm to normal cells and enhancing the safety and controllability of CAR-T therapy.
Fifth Generation CAR:
The fifth-generation CAR-T, known as universal CAR-T, achieves T-cell receptor α (TCR-α) and β (TCR-β) chain deletion by knocking out the TRAC gene. This implies the removal of the T-cell receptor (TCR) from the surface of T cells, thereby avoiding the occurrence of graft-versus-host disease (GVHD) in transplantation reactions.
Since the FDA’s approval of the CD19 CAR-T product, Novartis’s Kymriah, in 2017, CAR-T cell therapy has entered a stage of rapid development. However, the currently approved and marketed products are all second-generation CAR-T therapies. There is still a long way to go for CAR-T to become widespread in the market.
Safety concerns constitute the primary challenge for CAR-T, such as off-target effects, cytokine release syndrome (CRS), and neurotoxicity (NTX). Currently available CAR-T products primarily focus on treating hematologic malignancies, with no major breakthroughs achieved yet in treating solid tumors.
In 2021, China’s NMPA approved three CAR-T products for marketing: FOSUNKITE’s Axicabtagene Ciloleucel injection, JW Therapeutics’s Relmacabtagene Autoleucel Injection, and the recently approved JUVENTAS’s Inaticabtagene Autoleucel Injection, all targeting CD19. Additionally, earlier this year, IASO Bio obtained approval for Equecabtagene Autoleucel Injection, targeting BCMA. While CAR-T targeting CD19 has shown effectiveness, its scope remains limited to B-cell-related hematologic malignancies. BCMA-targeted CAR-T is restricted to treating multiple myeloma. To address solid tumor treatment, the development of more specific and potent targets is necessary.
Among the recently released domestically developed JUVENTAS’s Inaticabtagene Autoleucel Injection, its competitive advantage lies in its price, which has decreased to below one million RMB(Approximately $140,000 US).
With the continuous advancement of molecular biology technologies, more breakthroughs are expected in CAR molecule design. This progression anticipates the development of safer and more efficient universal CAR-T therapies in the future, benefiting a broader spectrum of cancer patients.
“If you’d like to inquire about the latest cancer-fighting technologies and treatments, you can contact us.”
whatsapp:+8613717959070
#CARTCellTherapy #CancerTreatment #ScienceInnovation #GeneticMedicine #TumorTreatment #HealthcareTech #MedicalScience #CancerAwareness #PatientCare #FutureOfMedicine
The new hope for pediatric patients with relapsed/refractory B-cell acute lymphoblastic leukemia (R/R B-ALL) boasts an overall survival rate of up to 96%.


