Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

**Breakthrough Chinese Monoclonal ADC Targets Nectin-4: Achieving 91.9% Disease Control Rate Across Multiple Solid Tumors**
**Breakthrough Chinese Monoclonal ADC Targets Nectin-4: Achieving 91.9% Disease Control Rate Across Multiple Solid Tumors**

Monoclonal ADC
#MonoclonalADC #Nectin4 #Solidtumor #9MW2821 #cervicalcancer #urothelialcarcinoma #esophagealcancer #breastcancer
Meet 9MW2821, a groundbreaking monoclonal antibody-drug conjugate (ADC) developed in China that targets Nectin-4, an adhesion molecule highly expressed in several solid tumors, including cervical cancer (CC), urothelial carcinoma (UC), esophageal cancer (EC), and breast cancer. Recently, this innovative therapy was granted Breakthrough Therapy Designation by the China Center for Drug Evaluation (CDE) for treating locally advanced or metastatic urothelial carcinoma, specifically in patients who have previously failed platinum-based chemotherapy and PD-(L)1 inhibitors.
The latest clinical results were unveiled at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, showcasing the remarkable efficacy of 9MW2821 in a Phase 1/2 trial involving 260 patients with various advanced solid tumors. These included UC, triple-negative breast cancer (TNBC), EC, and CC. Among 37 evaluable UC patients, the disease control rate (DCR) soared to an impressive 91.9%, with an objective response rate (ORR) of 62.2%. Additionally, the median overall survival (mOS) reached 14.2 months, while the median progression-free survival (mPFS) was 8.8 months.
This promising therapy is poised to redefine treatment paradigms for patients with advanced solid tumors who have limited options, marking a significant milestone in the global oncology landscape.

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp:+8613717959070
Email: doctor.huang@globecancer.com
#OncologyBreakthrough #CancerTreatment #Nectin4 #ADC #ClinicalResearch #Immunotherapy #UC #TNBC #CancerAwareness #BiotechInnovation
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

**A Groundbreaking ADC Drug for Cervical Cancer Approved in China Macau**
**A Groundbreaking ADC Drug for Cervical Cancer Approved in China Macau**

Cervical Cancer
On August 6, 2024, Zai Lab proudly announced the approval of TIVDAK® (tisotumab vedotin-tftv) in Macau, marking the world’s first ADC (antibody-drug conjugate) specifically for cervical cancer. This innovative treatment is intended for patients with recurrent or metastatic cervical cancer who have experienced disease progression during or after chemotherapy.
TIVDAK stands as a significant milestone, being the first ADC drug approved by the FDA for cervical cancer. Following its accelerated approval in 2021, the FDA granted full approval in April 2024, solidifying its use for patients needing treatment after chemotherapy failure.
The FDA’s full approval was backed by positive outcomes from the global Phase 3 clinical trial, innovaTV 301. This study demonstrated that patients treated with TIVDAK experienced an overall survival benefit compared to those receiving chemotherapy. This finding is particularly noteworthy, as TIVDAK is the first ADC drug globally to show clear survival benefits for patients with second and third-line metastatic or recurrent cervical cancer.
Cervical cancer remains a leading threat in women’s health. Despite advancements in prevention through vaccination and early diagnosis via screening, there remains a significant unmet need for effective treatments. In China alone, an estimated 150,000 new cases are diagnosed each year, with approximately 60,000 fatalities. At the time of diagnosis, around 15% of adult patients have metastatic disease. For those diagnosed early and receiving treatment, up to 61% experience recurrence.
Zai Lab is participating in the global innovaTV 301 study and anticipates submitting a marketing application in China by early 2025. This approval in Macau represents a significant step forward in providing new hope for cervical cancer patients worldwide.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#CervicalCancer #ADCDrug #TIVDAK #ZaiLab #CancerTreatment #WomenHealth #MedicalAdvancement #FDAApproval #ClinicalTrial #Macau #GlobalHealth #InnovativeTherapy #PatientCare #Pharmaceuticals
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
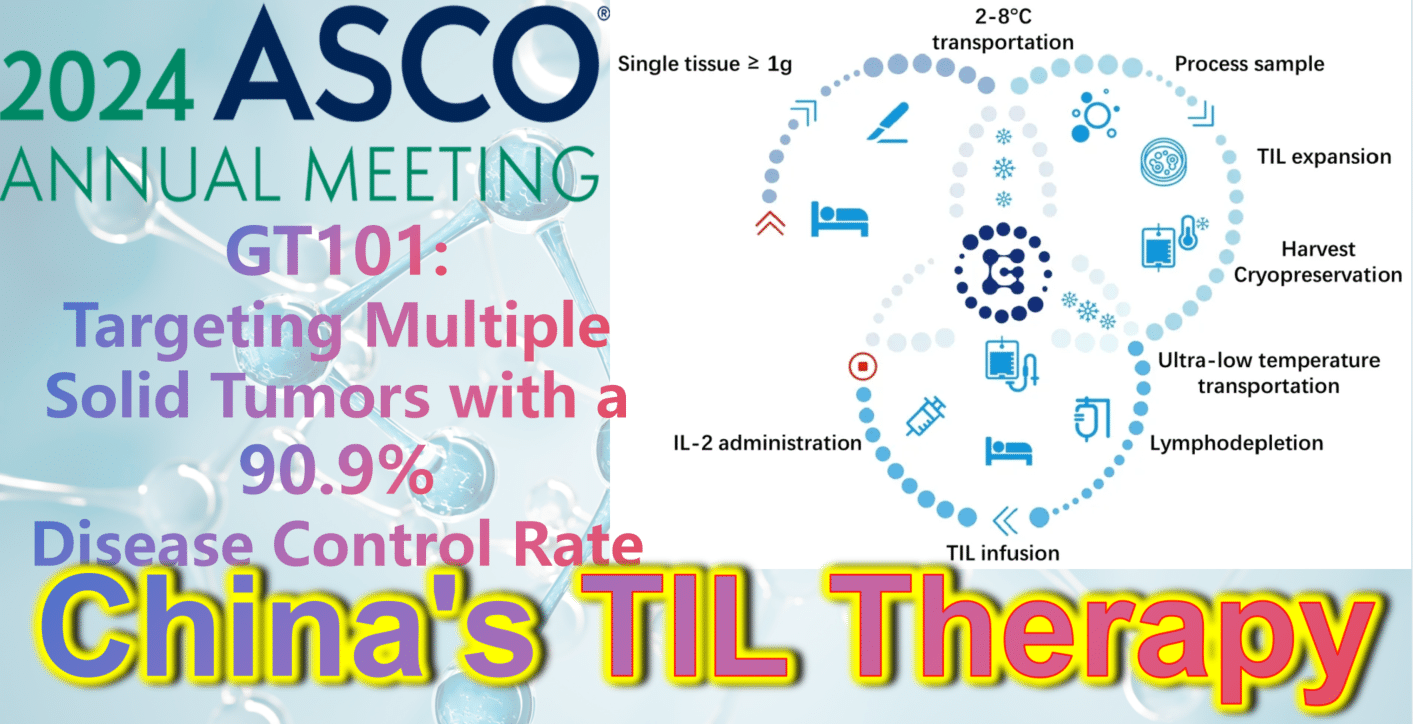
2024 ASCO China Highlights: China’s Indigenous TIL Therapy – GT101: Targeting Multiple Solid Tumors with a 90.9% Disease Control Rate
**2024 ASCO China Highlights: China’s Indigenous TIL Therapy Makes a Strong Debut, Targeting Small Cell Lung Cancer, Melanoma, Cervical Cancer, with a DCR Over 90%**
**GT101: Targeting Multiple Solid Tumors with a 90.9% Disease Control Rate**

TIL Therapy
GT101, independently developed by Gravel Biotech, is an autologous tumor-infiltrating lymphocyte (TIL) therapy. On April 22, 2022, its clinical trial implied consent was approved by the National Medical Products Administration (NMPA) (Acceptance No.: CXSL2200061). It is indicated for treating various solid tumors including non-small cell lung cancer, melanoma, and cervical cancer. Notably, GT101 is China’s first approved clinical TIL cell therapy and holds promise as the first cell therapy to conquer solid tumors!
Recently, at the 2024 ASCO Annual Meeting, results from the Phase 1 clinical trial of GT101 TIL therapy (NCT05430373) were announced. By November 10, 2023, a total of 14 patients with recurrent or metastatic solid tumors (including small cell lung cancer, melanoma, cervical cancer) had been enrolled, all with an ECOG performance status of 0 or 1 and having received a median of 2.6 prior lines of therapy. After enrollment, tumor tissue was obtained via appropriate surgical procedures for GT101 preparation, followed by approximately 30 days of TIL cell culture. Patients then underwent non-myeloablative lymphocyte depletion (cyclophosphamide + fludarabine), GT101 reinfusion therapy, and IL-2 (Interleukin-2) treatment.
1. **All enrolled patients (n=14):** The Objective Response Rate (ORR) was 35.7%. Among them, 28.6% (4 patients) achieved partial response (PR), 57.1% (8 patients) achieved stable disease (SD), and 7.1% (1 patient) achieved complete response (CR).
2. **In cervical cancer patients (n=11):** The Objective Response Rate (ORR) reached 45.5% (5/11). The Disease Control Rate (DCR) was as high as 90.9% (10/11), with 36.4% (4 patients) achieving partial response (PR) and 9.1% (1 patient) achieving complete response (CR). The median Progression-Free Survival (PFS) was 4.2 months. According to Kaplan-Meier statistics, the durations of complete response (CR) and progression-free survival (PFS) were 24 weeks and 36 weeks, respectively.
In conclusion, GT101 demonstrated promising clinical efficacy and manageable safety in combination with lymphocyte depletion and high-dose IL-2 treatment. Particularly in the treatment of cervical cancer, its objective response rate and duration of response are remarkable!
**How to Seek Help from TIL Therapy?**
The good news is that several TIL therapy clinical trials are currently recruiting in China, primarily targeting various solid tumors such as non-small cell lung cancer, melanoma, cholangiocarcinoma, esophageal squamous cell carcinoma, head and neck squamous cell carcinoma, breast cancer, ovarian cancer, cervical cancer, endometrial cancer, fallopian tube cancer, urothelial cancer, and renal cancer.
Patients seeking help from TIL therapy can submit their complete treatment history, recent pathology reports, imaging examination reports, and discharge summaries to Advanced Medicine in China
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#ASCO2024 #TILTherapy #GT101 #CancerResearch #SolidTumors #ClinicalTrials #LungCancer #Melanoma #CervicalCancer #Immunotherapy #ChinaBiotech #CancerTreatment
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese Hengrui Pharmaceuticals ‘s indication for cervical cancer has been approved for clinical trials.
🌟 Chinese Hengrui Pharmaceuticals ‘s indication for cervical cancer has been approved for clinical trials.🌟

cervical cancer
🏥💊(Jiangsu Hengrui Pharmaceuticals Co., Ltd)Hengrui Pharmaceutical’s PD-L1 inhibitor Atezolizumab injection combined with concurrent chemoradiotherapy for the treatment of locally advanced cervical cancer has been approved for open, multicenter Phase II clinical trials.

Atezolizumab
🌸🎀#Cervical cancer is the fourth most common cancer in women globally, mainly caused by persistent infection with high-risk human papillomavirus (#HPV). Although the widespread implementation of #cervicalcancer screening and the availability of HPV vaccines have reduced the incidence of cervical cancer in developed countries, it remains one of the common malignant tumors in women.
🏥💊Atezolizumab injection is a humanized anti-PD-L1 monoclonal antibody independently developed by Hengrui Medicine. It is the first PD-L1 inhibitor approved for the treatment of small cell lung cancer in China. By specifically binding to the PD-L1 molecule, it blocks the PD-1/PD-L1 pathway that leads to tumor immune tolerance, reactivating the immune system’s anti-tumor activity and achieving the goal of tumor treatment.

The Lancet Oncology
🌸🎀Atezolizumab injection has been studied in various fields, including small cell lung cancer, #NSCLC non-small cell lung cancer, esophageal cancer, liver cancer, and cervical cancer. It has shown promising efficacy in small cell lung cancer. Based on the results of the SHR-1316-III-301 study, the application for the market approval of Atezolizumab injection combined with chemotherapy as first-line treatment for extensive-stage small cell lung cancer has been accepted and approved in China in March 2023. The study results have been published in the top international medical journal “The Lancet Oncology,” and the original research from China has been internationally recognized.
🏢💊Jiangsu Hengrui Pharmaceuticals Co., Ltd is committed to advancing the clinical research of the PD-L1 inhibitor Atezolizumab injection, bringing more innovative and effective treatment options for cervical cancer patients. Stay tuned for our progress!

Hengrui Pharmaceutical
🚑In addition to Cervical cancer, we are currently urgently recruiting patients with B-cell lymphoma, T-cell lymphoma, T-cell leukemia (T-ALL), acute lymphoblastic leukemia, myeloma, and other types of cancer!
You can send electronic copies or photos of genetic testing reports and diagnostic reports to the 📩email address: doctor.huang@globecancer.com📩, or click on the ✉️WhatsApp+8613717959070✉️ icon on the homepage. The Medical Department will contact you as soon as they receive the reports.
#CervicalCancerTreatment #Atezolizumab #HengruiMedicine #ClinicalTrials #CancerTreatment #cervical #cervicalcancer #chinesemedicine #newdrug #Lancet #TheLancetOncology
📩References:
[1]gco.iarc.fr(WHO statistics)
[2]Rose PG,Bundy BN,Watkins EB,Thigpen JT,Deppe G,Maiman MA,Clarke-Pearson DL,Insalaco S:Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer.N Engl J Med1999,340(15):1144-1153.
[3]Pfaendler KS,Tewari KS:Changing paradigms in the systemic treatment of advanced cervical cancer.Am J Obstet Gynecol2016,214(1):22-30.
[4]Liu C,Lu J,Tian H,Du W,Zhao L,Feng J,Yuan D,Li Z:Increased expression of PDL1by the human papillomavirus16E7oncoprotein inhibits anticancer immunity.Mol Med Rep2017,15(3):1063-1070.
