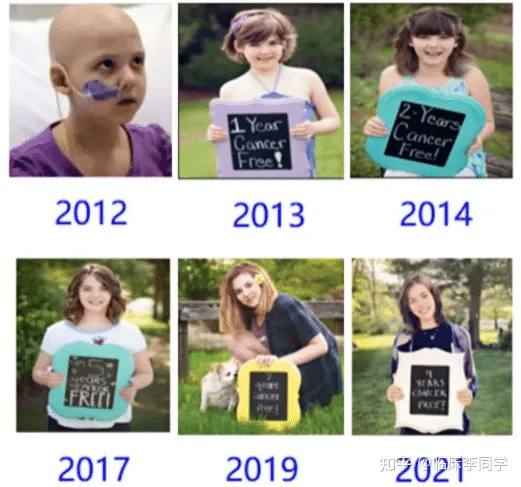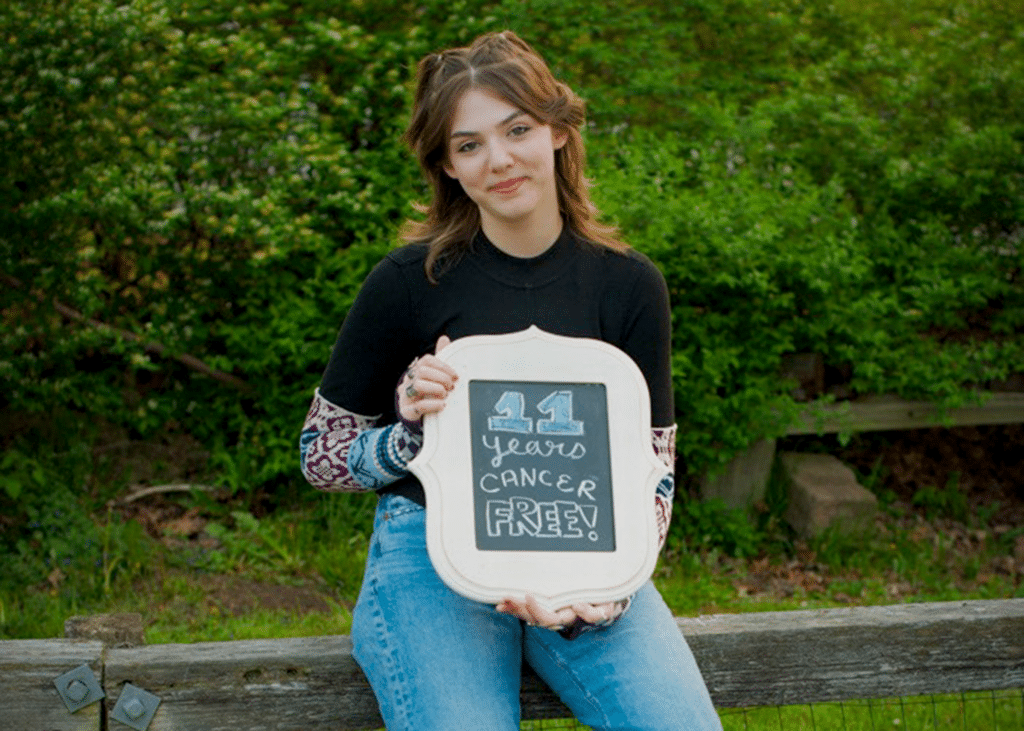Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Clinical Breakthrough: Chinese CAR-T – Inaticabtagene Autoleucel Revolutionizing Hematologic Cancer Therapy


Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
Breakthrough Advances in CAR-T Cell Therapy Combined with TKI for Malignant Hematologic System Tumors



Unveiling Hope: Chinese CAR-T Cell Therapy Illuminating New Paths in Liver Cancer Treatment!
2020, over 19.3 million people were diagnosed with cancer worldwide, leading to almost 10 million fatalities. In China alone, the number of new cancer patients reached a staggering 4.57 million, accounting for 23.7% globally. Liver cancer, among the most prevalent malignant tumors in China, witnessed 410,000 new cases and 380,000 deaths, making up 45.3% and 47.1% of the global total, respectively [1]. However, since the 21st century began, significant strides have been made in liver cancer treatments, particularly in medication and localized therapies. Surgical procedures are no longer the sole option for long-term survival among liver cancer patients.
Immunotherapy has emerged as one of the most promising techniques for treating liver cancer, especially with advancements in tumor molecular biology. In 2013, “Science” magazine categorized immunotherapy as the fourth major cancer treatment, following surgery, chemotherapy, and radiation therapy, with cell therapy becoming a focal point of basic and clinical research in recent years.

In May 2020, Professor Zhai Bo’s team from Shanghai Jiao Tong University School of Medicine’s Renji Hospital, in collaboration with Shanghai Sci-Tech Biotechnology’s team led by Li Zonghai, published groundbreaking preliminary clinical research data on CAR-T cell therapy targeting the GPC3 gene for hepatocellular carcinoma in the “Clinical Cancer Research” journal. This breakthrough study brought unprecedented hope for CAR-T cell therapy in liver cancer treatment.

Even more inspiring, their publication in the “Cancer Communications” journal showcased follow-up results of two late-stage liver cancer patients who achieved long-term tumor-free survival after receiving CAR-T cell combined with local therapy [2]. These findings shed new light on the treatment prospects for liver cancer patients.
However, despite the potential therapeutic effects of liver cancer CAR-T cell therapy, it faces challenges and obstacles. Liver cancer’s heterogeneity, tumor microenvironment, and the safety of cell therapy remain crucial issues to address.
Presently, revolutionary changes are underway in the treatment models and concepts for liver cancer. However, integrating CAR-T cell therapy into actual liver cancer treatment requires further scientific exploration and clinical research. Researchers emphasize that only through comprehensive utilization of CAR-T cells in conjunction with other treatment modalities can its therapeutic potential be fully realized.
Professor Zhai Bo’s team is currently conducting various fundamental and clinical studies aimed at exploring additional possibilities for CAR-T cell therapy in solid tumors. These studies include phase I clinical research on EpCAM CAR-T cell combined with ablative therapy for gastrointestinal tumors, phase II clinical research on Claudin18.2 CAR-T cell therapy for gastrointestinal tumors, studies on the mechanism and prevention of OTOT toxicity, among others. These endeavors will provide more experimental data and support for the application of CAR-T cells in the treatment of solid tumors.
In conclusion, liver cancer CAR-T cell therapy signifies a significant breakthrough in the field of liver cancer treatment, offering new hope for patients. Despite the challenges to overcome, the outlook for this therapy is promising and holds the potential to bring a blessing to more patients in the future.
[References]
Zhaibo, Lizonghai etc.
“Chimeric Antigen Receptor-Glypican-3 T-Cell Therapy for Advanced Hepatocellular Carcinoma: Results of Phase 1 Trials.” 《Clinical Cancer Research》, 2020.
“Combined local therapy and CAR-GPC3 T-cell therapy in advanced hepatocellular carcinoma: a proof-of-concept treatment strategy.” 《Cancer Communications》, 2023.
#HealthTech#CancerResearch #Immunotherapy #CARTcell #LiverCancer #MedicalBreakthrough #ClinicalTrials #ScienceNews #HealthcareInnovation #ResearchBreakthrough #MedicalScience #CancerTherapy #CancerAwareness #InnovativeMedicine #ImmunotherapyTreatment #ScienceUpdates #HealthcareTechnology #BiomedicalResearch #ClinicalInnovation #CancerTreatment #MedicalAdvancements #ImmunologyResearch #HealthcareIndustry #ProfessionalHealthcare
The new hope for pediatric patients with relapsed/refractory B-cell acute lymphoblastic leukemia (R/R B-ALL) boasts an overall survival rate of up to 96%.


If a child unfortunately gets leukemia, should CAR-T Therapy be considered?