Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
China’s new drug Aumolertinib Mesilate Tablets: The world’s second EGFR-targeted therapy for lung cancer with gene mutations.
China’s new drug Aumolertinib Mesilate Tablets: The world’s second EGFR-targeted therapy for lung cancer with gene mutations.

lung cancer
#LungCancer #EGFRInhibitor #TargetedTherapy #Aumolertinib #NSCLC
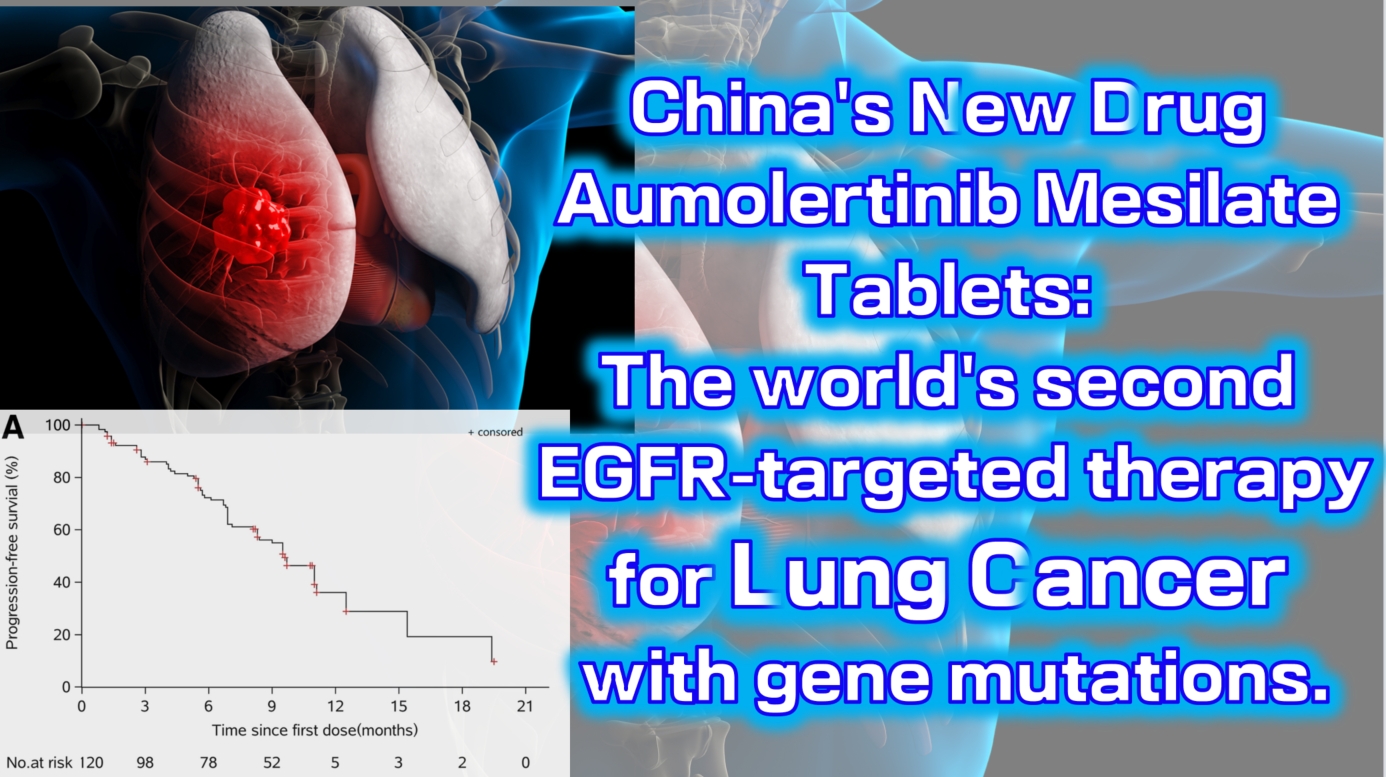
Aumolertinib Mesilate Tablets (Ameile) were approved for marketing in China on March 18, 2020. It is China’s first third-generation EGFR-targeted therapy for lung cancer with gene mutations and the second such drug globally, following Osimertinib.
On August 20, 2024, the new indication marketing application for Aumolertinib Mesilate Tablets was accepted for priority review by the China Center for Drug Evaluation (CDE). This indication is for the treatment of unresectable locally advanced non-small cell lung cancer (NSCLC) that has not progressed following platinum-based chemoradiotherapy. This is the fourth indication for which the drug has submitted a marketing application, and it is expected to be approved in the second quarter of 2025.
The Phase 1 clinical trial (NCT0298110) enrolled a total of 120 patients with locally advanced or metastatic non-small cell lung cancer (NSCLC). The results showed an objective response rate (ORR) of 52% (range: 42–63), with a disease control rate (DCR) as high as 92% (range: 84–96). The median progression-free survival was 11.0 months (95% CI: 9.5-not reached).

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#CancerTreatment #ClinicalTrials #PharmaInnovation #Oncology #DrugApproval #Biotech #PrecisionMedicine
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese Exciting Good News Update in Lung Cancer Treatment! ORR: 78.6%
 Chinese Exciting Good News Update in Lung Cancer Treatment! ORR: 78.6%
Chinese Exciting Good News Update in Lung Cancer Treatment! ORR: 78.6%
 Meet Sunvozertinib, the game-changer in advanced lung cancer therapy!
Meet Sunvozertinib, the game-changer in advanced lung cancer therapy! 

Sunvozertinib, NSCLC
At the 2023 ESMO Conference, fresh data from early trials of Sunvozertinib’s frontline treatment for EGFR exon20ins-mutated advanced NSCLC were unveiled, and the results were jaw-dropping! 
 With an astounding Objective Response Rate (ORR) of 78.6% and a median Progression-Free Survival (PFS) of 12.4 months at the recommended dose (300mg QD), Sunvozertinib once again set a new benchmark in this field, offering a superior treatment option for newly diagnosed patients with EGFR exon20ins mutations!
With an astounding Objective Response Rate (ORR) of 78.6% and a median Progression-Free Survival (PFS) of 12.4 months at the recommended dose (300mg QD), Sunvozertinib once again set a new benchmark in this field, offering a superior treatment option for newly diagnosed patients with EGFR exon20ins mutations! 

Let’s dive into a real success story: A 35-year-old male diagnosed with late-stage NSCLC harboring EGFR exon20ins mutations, along with metastases to the brain, liver, and bones. After showing stable disease (SD) following two cycles of platinum-based chemotherapy, he switched to Sunvozertinib. Just one month into treatment, a follow-up chest CT scan revealed a remarkable 34% reduction in the size of the left lower lobe lesion, with lung lesions shrinking from 29mm×26mm to 19mm×15mm (see image). Multiple nodules in the lungs and pleura also decreased in size, along with liver lesions. Evaluation: Partial Response (PR)! 


Lung Cancer
Noteworthy is the impeccable safety profile of Sunvozertinib, with no significant adverse effects reported during treatment. As of this post, the patient continues to receive Sunvozertinib monotherapy with promising outcomes! 

With breakthroughs in targeted therapies for EGFR exon20ins-mutated NSCLC, several new drugs are actively exploring frontline treatment options. The early study results of Sunvozertinib’s frontline therapy for EGFR exon20ins-mutated advanced NSCLC have once again rewritten the records in this field, providing new treatment guidelines for these patients! 

Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China’s Breakthrough: Sunvozertinib Leads the Wave of New Drug Development in EGFR exon20ins Mutant NSCLC
🌈🌈China’s Breakthrough: Sunvozertinib Leads the Wave of New Drug Development in EGFR exon20ins Mutant NSCLC🌸🌸
**Caption:**
🇨🇳 Exciting news from China’s research! Sunvozertinib, a novel drug by Dizal Pharmaceuticals, is making strides in treating EGFR exon20ins mutant NSCLC. 🌐✨

Sunvozertinib
**Article:**
Dizal Pharmaceuticals’ EGFR tyrosine kinase inhibitor (TKI) Sunvozertinib, known as 舒沃替尼 (Shuwo Tini) in China, has achieved a significant milestone with the publication of data from its Chinese registration study (WU-KONG6) in “The Lancet Respiratory Medicine.” This study validates the clinical efficacy of the Chinese-developed drug in treating late-stage non-small cell lung cancer (NSCLC) with EGFR exon20ins mutations, marking a crucial breakthrough for patients who have long faced challenges in finding effective treatments.
**Challenges in Treating EGFR exon20ins Mutant NSCLC**
EGFR exon20ins mutation has been a formidable subtype in EGFR-mutant NSCLC, with limited effectiveness observed in traditional EGFR-TKI and chemotherapy treatments. Faced with this challenge, pharmaceutical companies are actively engaged in new drug development to propel advancements in the treatment landscape for EGFR exon20ins mutant NSCLC. The success of the WU-KONG6 study signifies a critical recognition in this field and affirms the high efficacy and low toxicity of Sunvozertinib in treating EGFR exon20i

Lancet
ns mutant NSCLC.
**Results from the Chinese Registration Study “WU-KONG6″**
The results of the Chinese registration study “WU-KONG6” demonstrate that Sunvozertinib achieved an objective response rate (ORR) of 60.8% in treated patients with EGFR exon20ins mutations. This indicates a significant tumor reduction in over 60% of patients, leading to a substantial decrease in tumor burden. Moreover, patients experienced improvements in clinical symptoms and quality of life. Notably, Sunvozertinib is currently the only drug that has elevated the ORR in treated patients with EGFR exon20ins mutations to over 50%, breaking previous treatment ceilings and showcasing its potential as the “best in class.”
**Looking Ahead**
The competition in the field of EGFR exon20ins mutant NSCLC treatment is intensifying, and the successful introduction of Sunvozertinib provides a new therapeutic option for patients. As other pharmaceutical companies globally advance in new drug development, the innovation and competition in this field are expected to escalate, bringing more hope to patients with EGFR exon20ins mutant NSCLC. In the continual pursuit of medical breakthroughs, the emergence of new drugs will offer more effective treatment options, contributing to the extension of patients’ survival periods.
#MedicalInnovation #CancerResearch #HopeForPatients #Sunvozertinib #MedicalBreakthrough #ChinaInnovation
