Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

🍀Чудесное путешествие через границы: триумф мистера Баранкова с китайской терапией CAR-T🍀
🍀Чудесное путешествие через границы: триумф мистера Баранкова с китайской терапией CAR-T🍀

ЧудесноеПутешествие
🌿Познакомьтесь с мистером Баранковым,
68-летним пациентом из Санкт-Петербурга, Россия, который внезапно был диагностирован с множественной миеломой в 2018 году. После четырех лет международных консультаций он обнаружил, что Китай значительно продвинулся в области терапии CAR-T по сравнению с европейскими медицинскими учреждениями в Испании, Мюнхене, Гейдельберге и других странах. Результаты лечения в Китае ведущие в мире.
🌱После четырех лет лечения и рецидивов
После неудачной традиционной химиотерапии и двух автологичных трансплантаций гемопоэтических стволовых клеток, у него появилась возможность провести онлайн-консультацию с экспертами Международной больницы Цзяхуи в Шанхае. Во время виртуальной консультации господин Баранков был впечатлен опытом китайской медицинской команды и потенциалом лечения с помощью полностью человеческого препарата CAR-T FUCASO (Eque-cel). Учитывая лидирующее положение Китая в области терапии CAR-T, более конкурентоспособные затраты и относительно короткое время ожидания, господин Баранков принял решение отправиться в Китай для получения лечения.
☘️
#ЧудесноеПутешествие #ТерапияCART #ПересечениеГраниц #MiracleJourney #CARTTherapy #CrossingBorders #CART #Hopeofpatients #FUCASOApproval #Equecel #MultipleMyeloma #JiahuiHospital #Shanghai #Immunotherapy #MedicalInnovation #MedicalBreakthrough #CancerTreatment #FullyHumanCART
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Exciting News Alert! China’s New TCR-T Product IND Approved for Synovial Sarcoma!
🌟 Exciting News Alert! China’s New TCR-T Product IND Approved for Synovial Sarcoma! 🌟

synovial sarcoma

Cell Rep Med
China’s first TCR-T product IND approval takes aim at synovial sarcoma.
TAEST16001,

TCR-T Therapy
How to Seek TCR-T Therapy Assistance
or click on the WhatsApp+8613717959070
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese Hengrui Pharmaceuticals ‘s indication for cervical cancer has been approved for clinical trials.
🌟 Chinese Hengrui Pharmaceuticals ‘s indication for cervical cancer has been approved for clinical trials.🌟

cervical cancer

Atezolizumab

The Lancet Oncology
🌸🎀Atezolizumab injection has been studied in various fields, including small cell lung cancer, #NSCLC non-small cell lung cancer, esophageal cancer, liver cancer, and cervical cancer. It has shown promising efficacy in small cell lung cancer. Based on the results of the SHR-1316-III-301 study, the application for the market approval of Atezolizumab injection combined with chemotherapy as first-line treatment for extensive-stage small cell lung cancer has been accepted and approved in China in March 2023. The study results have been published in the top international medical journal “The Lancet Oncology,” and the original research from China has been internationally recognized.

Hengrui Pharmaceutical
🚑In addition to Cervical cancer, we are currently urgently recruiting patients with B-cell lymphoma, T-cell lymphoma, T-cell leukemia (T-ALL), acute lymphoblastic leukemia, myeloma, and other types of cancer!
You can send electronic copies or photos of genetic testing reports and diagnostic reports to the 📩email address: doctor.huang@globecancer.com📩, or click on the ✉️WhatsApp+8613717959070✉️ icon on the homepage. The Medical Department will contact you as soon as they receive the reports.
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese Exciting Good News Update in Lung Cancer Treatment! ORR: 78.6%
 Chinese Exciting Good News Update in Lung Cancer Treatment! ORR: 78.6%
Chinese Exciting Good News Update in Lung Cancer Treatment! ORR: 78.6%
 Meet Sunvozertinib, the game-changer in advanced lung cancer therapy!
Meet Sunvozertinib, the game-changer in advanced lung cancer therapy! 

Sunvozertinib, NSCLC

Lung Cancer
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Breakthrough in Cancer Treatment in CHINA: TX103 CAR-T Approved for Clinical Use Against Recurrent Brain Gliomas
🔬”Breakthrough in Cancer Treatment in CHINA: TX103 CAR-T Approved for Clinical Use Against Recurrent Brain Gliomas”🔬🧠

Brain Gliomas
✨ Exciting news in cancer research! Fuzhou Tuoxin Tiancheng Biotech’s TX103 CAR-T, a revolutionary therapy for recurrent stage 4 gliomas, has received clinical approval. 🌟 🧠
Article:

Tuoxin Tiancheng
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Breakthrough in Reducing Lupus with Successful Application of CAR-T Dual Target Technology
🌈Breakthrough in Reducing Lupus with Successful Application of CAR-T Dual Target Technology 🌟

Lupus

Lupus
🙌 Yin expressed her gratitude, saying: “The red spots on my body have disappeared, and I no longer need hormone medications or immunosuppressants. My mental state is also great, and all indicators are excellent. Today is my third checkup, and the results of the first two were also very positive. I thank these healthcare professionals for giving me a second life.”
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Revolutionizing Medicine: CAR-T Therapy Beyond Cancer
🚀Revolutionizing Medicine: CAR-T Therapy Beyond Cancer 🚀

🔬 Over the past decade, CAR-T cell therapy has transformed the field of oncology, successfully treating previously incurable blood cancers. While CAR-T therapy gained fame for its success in cancer treatment, the roots of this groundbreaking principle trace back nearly 30 years—initially exploring T cell therapy for HIV/AIDS. Although the early attempts didn’t succeed in treating HIV/AIDS, they demonstrated the enduring potential of engineered T cells in immunocompromised patients.
🚀 The future of CAR-T therapy holds vast potential in reshaping the landscape of medical treatment, reaching far beyond the realms of cancer. Stay tuned for ground
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Exciting breakthrough in Chinese pharmaceutical research!
[🔬🌏] Exciting breakthrough in Chinese pharmaceutical research!

Chinese medicial research
💊Since 2019, China has witnessed a surge in phase 1 and 2 clinical trials, with a focus on addressing prevalent cancers like stomach, esophageal, and cervical cancers. The world is now turning its attention to China’s rapid emergence on the global stage of pharmaceutical innovation.
🌟 Key Highlights:
🚀 2020 and Beyond:
🌿 Traditional Medicine Meets Innovation:
🔍 Future of Cellular Therapy:
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China Leading Global Advances in Breast Cancer Immunotherapy
🌟China Leading Global Advances in Breast Cancer Immunotherapy 🌟#ChinaInMedicine #BreastCancer

Breast Cancer
Since 2020, breast cancer has surpassed lung cancer, becoming the most common cancer globally with approximately 2.3 million new cases and 680,000 deaths annually. Despite improvements in traditional treatments like surgery, chemotherapy, and radiation, the mortality rate remains high. China’s Chimeric Antigen Receptor (CAR) immunotherapy is making significant strides, spearheading innovation in breast cancer treatment. 🇨🇳💪
China is not only a trailblazer in breast cancer immunotherapy research but also a miracle creator, bringing new hope to cancer patients worldwide. Let’s witness China’s outstanding achievements in the medical field and acknowledge its contribution to global health! 💊🌏

Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
Chinese Research: Hopes of CAR-T in Second-Line Treatment of LBCL(Lymphoma)


The Emergence of Fifth Generation CAR-T: A Boon for Late-Stage Cancer Patients or a Major Breakthrough in Solid Tumor Treatment?
The fifth-generation CAR-T is designed as a universal type of CAR-T. Is this risk-free CAR-T capable of achieving significant breakthroughs in solid tumor treatment, or is it effectively reducing costs to enable scalable production and treatment?
After nearly three decades of development, CAR (Chimeric Antigen Receptor) technology has undergone continuous innovation. Currently, CAR has evolved to its fifth generation. Its aim is to enhance the safety of treatments by reducing toxicity and non-specific antigen recognition. This is achieved by stimulating proliferation, activation, and the generation of memory phenotypes within CAR-T cells to improve efficiency and provide immune regulation for the optimal function of CAR-T cells.
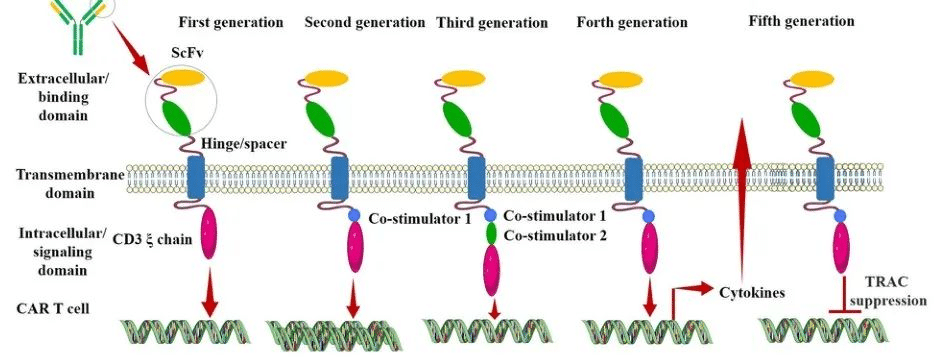
Generation CAR-T
The Evolution of Different Generations of CAR-T
First Generation CAR:
The first-generation CAR comprises an extracellular single-chain variable fragment (scFv) as the antigen recognition binding domain and an intracellular CD3ζ as the cellular activation signaling domain. Despite initiating cytotoxic anti-tumor responses within transplanted T cells, first-generation CAR-T cells exhibit lower levels of cytotoxicity and proliferation due to the CAR structure lacking co-stimulatory domains, which results in inadequate interleukin (IL)-2 production.
Second Generation CAR:
Building upon the CD3ζ signal transduction domain, the second-generation CAR includes an additional co-stimulatory signaling domain that activates T cells, significantly enhancing T cell proliferation and survival. For instance, CD28 can deliver robust activation signals, enabling T cells to achieve high levels of cytotoxic activity in a shorter duration, while 4-1BB provides prolonged activation signals, sustaining T cell-mediated killing of tumor cells. However, limitations arise in second-generation CAR-T cells utilizing retroviruses as viral vectors, restricting the length of transgene fragments they can carry. As a result, it becomes necessary to choose between incorporating CD28 and 4-1BB into T lymphocytes.
Third Generation CAR:
Third-generation CAR-T cells utilize larger DNA-carrying lentiviruses as viral vectors, allowing simultaneous incorporation of DNA fragments for both CD28 and 4-1BB into T cells. Consequently, the third-generation CAR structure encompasses two co-stimulatory domains, theoretically addressing the need for higher activation intensity and sustained survival of CAR-T cells. However, the safety concerns associated with prolonged and high-level persistence of CAR-T cells, including potential attacks on the host’s immune system, remain unresolved despite these advancements.
Fourth Generation CAR:
The design concept behind the fourth-generation CAR revolves around the precise treatment of cancerous diseases. For instance, solid tumors generate a microenvironment (TME) during their chronic progression, preventing CAR-T cells from penetrating the tumor interior. As a result, CAR-T therapy demonstrates limited efficacy in treating solid tumors. TRUCK CAR-T involves incorporating cytokines (such as IL-12) or chemokines into the CAR structure. This facilitates increased infiltration of T cells into tumor tissues while recruiting other immune cells within the body to eliminate tumor cells. In some studies, a suicide gene or certain drug-sensitive genes are attached to the CAR structure to ensure the clearance of CAR-T cells from the body post-treatment, preventing inadvertent harm to normal cells and enhancing the safety and controllability of CAR-T therapy.
Fifth Generation CAR:
The fifth-generation CAR-T, known as universal CAR-T, achieves T-cell receptor α (TCR-α) and β (TCR-β) chain deletion by knocking out the TRAC gene. This implies the removal of the T-cell receptor (TCR) from the surface of T cells, thereby avoiding the occurrence of graft-versus-host disease (GVHD) in transplantation reactions.
Since the FDA’s approval of the CD19 CAR-T product, Novartis’s Kymriah, in 2017, CAR-T cell therapy has entered a stage of rapid development. However, the currently approved and marketed products are all second-generation CAR-T therapies. There is still a long way to go for CAR-T to become widespread in the market.
Safety concerns constitute the primary challenge for CAR-T, such as off-target effects, cytokine release syndrome (CRS), and neurotoxicity (NTX). Currently available CAR-T products primarily focus on treating hematologic malignancies, with no major breakthroughs achieved yet in treating solid tumors.
In 2021, China’s NMPA approved three CAR-T products for marketing: FOSUNKITE’s Axicabtagene Ciloleucel injection, JW Therapeutics’s Relmacabtagene Autoleucel Injection, and the recently approved JUVENTAS’s Inaticabtagene Autoleucel Injection, all targeting CD19. Additionally, earlier this year, IASO Bio obtained approval for Equecabtagene Autoleucel Injection, targeting BCMA. While CAR-T targeting CD19 has shown effectiveness, its scope remains limited to B-cell-related hematologic malignancies. BCMA-targeted CAR-T is restricted to treating multiple myeloma. To address solid tumor treatment, the development of more specific and potent targets is necessary.
Among the recently released domestically developed JUVENTAS’s Inaticabtagene Autoleucel Injection, its competitive advantage lies in its price, which has decreased to below one million RMB(Approximately $140,000 US).
With the continuous advancement of molecular biology technologies, more breakthroughs are expected in CAR molecule design. This progression anticipates the development of safer and more efficient universal CAR-T therapies in the future, benefiting a broader spectrum of cancer patients.
“If you’d like to inquire about the latest cancer-fighting technologies and treatments, you can contact us.”
whatsapp:+8613717959070
#CARTCellTherapy #CancerTreatment #ScienceInnovation #GeneticMedicine #TumorTreatment #HealthcareTech #MedicalScience #CancerAwareness #PatientCare #FutureOfMedicine
