Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

CAR-T Cell Therapy as a New Option for Treating Multiple Myeloma
**CAR-T Cell Therapy as a New Option for Treating Multiple Myeloma**

Multiple Myeloma
“CAR-T cell therapy has now become a new option for treating patients with relapsed and refractory multiple myeloma, with hopes of changing the difficult situation in myeloma treatment where treatments are either ineffective or unavailable,” said Jin Jie, Director of the Hematology Department at the First Affiliated Hospital of Zhejiang University School of Medicine, in an interview with People’s Daily Health on October 23.
Early on October 23, Jin Jie visited the hospital ward to see a 72-year-old patient with multiple myeloma. Just the day before, the patient had begun receiving treatment with a domestically produced BCMA-targeting CAR-T drug.
“This 72-year-old patient came to the hospital due to bone pain and was even unable to walk steadily,” Jin recalled. After being diagnosed with multiple myeloma, the patient initially received a series of chemotherapy regimens that showed some efficacy. However, a year after undergoing autologous hematopoietic stem cell transplantation, the disease relapsed, and various subsequent treatments proved suboptimal. As a result, the patient opted for CAR-T therapy.
Since the approval of Equecabtagene Autoleucel last year, Professor Jin Jie became one of the first doctors nationwide to prescribe CAR-T drugs for multiple myeloma. This patient is her tenth multiple myeloma patient to receive this innovative therapy. Discussing the treatment outcomes, Jin noted, “The overall efficacy for patients has been very good.” A month ago, another patient who underwent this CAR-T therapy returned for a follow-up. “Everything was going well; we will continue to monitor the patient’s indicators until all data are stable.”
Multiple myeloma is a malignant plasma cell disease that predominantly affects older adults. Jin observed that the incidence of multiple myeloma has been trending younger in recent years, with patients in their forties commonly seen in clinical settings. Currently, multiple myeloma remains an incurable disease, and most patients face inevitable relapse. However, thanks to the emergence of new drugs and advances in treatment, both the efficacy and overall survival of patients have significantly improved in recent years.
“CAR-T therapy involves collecting a patient’s T cells and equipping them with a ‘weapon.’ These T cells are then expanded outside the body and re-infused into the patient, where they target and attack tumor cells, while also being able to proliferate in the patient’s body to eliminate cancer cells,” Jin explained. CAR-T therapy has the potential to give patients a higher quality of life.
CAR-T cell therapy has been advancing rapidly in the field of multiple myeloma, with multiple products already approved and available. In Jin’s view, patients should, under professional guidance, choose drugs and treatments with extensive clinical experience and proven efficacy.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
**#MultipleMyeloma #CAR_TCellTherapy #CancerTreatment #Immunotherapy #Hematology #CellTherapy #MedicalInnovation #PatientCare #Oncology #ZhejiangUniversity**
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

What Kind of Multiple Myeloma Patients Are Suitable for CAR-T Therapy in China?
# What Kind of Multiple Myeloma Patients Are Suitable for CAR-T Therapy in China?

Multiple Myeloma
#MultipleMyeloma #CAR_Therapy #MM #CART #RRMM #Hematology
**Multiple Myeloma** (MM) is a malignant hematological disease that affects plasma cells. In recent years, various drugs and treatment methods, such as proteasome inhibitors, immunomodulatory drugs, and autologous stem cell transplantation, have significantly improved the prognosis of multiple myeloma patients. However, a considerable number of patients still experience relapses and refractory disease. For these patients, CAR-T cell therapy is emerging as a groundbreaking and effective treatment option.
China has made remarkable progress in CAR-T therapy technology in recent years, becoming a global leader in the field of hematological diseases. This has attracted patients worldwide to seek this advanced treatment. So, which multiple myeloma patients are suitable to receive CAR-T treatment in China?
## 1. **Indications: Preferred Choice for Drug-Resistant/Relapsed/Refractory Multiple Myeloma Patients**
CAR-T therapy is typically recommended for multiple myeloma patients who have developed resistance to standard treatments (such as proteasome inhibitors, immunomodulatory drugs, and monoclonal antibodies) and are in their third line of treatment or beyond. In China, CAR-T therapy has broader indications and better efficacy.
### Suitable Patient Types:
-
Relapsed or refractory multiple myeloma patients, typically those who have undergone at least second-line treatments.
-
Patients with high-risk genetic characteristics, such as certain mutations (e.g., 17p deletion, t(4;14) translocation).
-
Patients unable to tolerate traditional treatments, including chemotherapy, other targeted therapies, stem cell transplantation, or immunomodulatory treatments, due to poor tolerance or severe side effects.
-
Patients who are BCMA-positive.
-
Patients whose disease continues to progress despite other treatments, especially chemotherapy.
-
Patients who are ineligible for stem cell transplantation.
-
Patients with severe symptoms not responsive to conventional treatments, such as bone pain, anemia, hypercalcemia, and kidney damage.
-
High tumor burden patients—experts generally reduce the tumor burden first before administering CAR-T therapy, often through bridging or sequential treatments.
Among the four global CAR-T products targeting the BCMA marker in multiple myeloma, two Chinese products stand out for their efficacy and affordability. The most effective product currently is IASO Bio’s FUCASO (Equecabtagene Autoleucel), with a complete response (CR) rate of 82.4%.
## 2. **Patients in Good Physical Condition**
CAR-T therapy is essentially a powerful immunotherapy. Although it has remarkable efficacy, it also carries some risks of side effects, including cytokine release syndrome (CRS) and neurotoxic reactions. China’s CAR-T treatment system is highly developed, with established treatment plans and consensus. The country has extensive experience in managing CRS, neurotoxins, and side effects, keeping the risks very low. Moreover, the expert doctors at Advanced Medicine in China have substantial experience in managing these risks. Nonetheless, patients are typically required to have stable physical health to withstand the risks associated with the treatment.
– Overall good physical function; patients with heart, lung, liver, or kidney dysfunction may require a secondary evaluation by an expert team.
– Patients need to score 0-1 on the ECOG performance status scale, indicating that they are capable of daily activities and self-care.
## 3. **Patients with Financial and Time Support for CAR-T Therapy**
CAR-T therapy’s high cost and complex manufacturing process require patients and their families to have financial support. CAR-T therapy in China is significantly more affordable than in Western countries. While CAR-T treatments in the U.S. may cost $600,000 to $700,000, China’s approved CAR-T treatments cost around $100,000, about one-fifth to one-seventh of U.S. prices. Nevertheless, it remains a high-cost treatment, so patients need to be fully aware of the expenses involved.
China has invested heavily in CAR-T research in recent years. Many top hospitals and research institutions conduct related clinical trials. China accounts for over 50% of global CAR-T clinical trials. If a patient qualifies for a clinical trial, participating in it could be a more economical option, offering access to the latest CAR-T therapies, sometimes costing only tens of thousands of dollars or even free.
The CAR-T treatment process is relatively long. It involves collecting T cells, modifying them, re-injecting them, and close monitoring, requiring the patient to have enough time and patience to complete the entire process. The fastest known case took two weeks to complete all steps and discharge with complete response (CR), but generally, the process takes about four weeks or longer, depending on the patient’s condition.
For patients who can afford the treatment and have the time to complete it, CAR-T therapy in China is undoubtedly an attractive option.
## Conclusion
China’s leading position in CAR-T cell therapy technology and lower treatment costs make it a popular destination for multiple myeloma patients worldwide. It offers new hope for relapsed or refractory multiple myeloma patients. By working with experienced medical teams, patients can receive more personalized treatment plans and benefit from the rapid development of CAR-T therapy globally.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CancerTreatment #ChinaMedicalInnovation #Immunotherapy #MyelomaTreatment #AdvancedMedicine #RelapsedMyeloma #CancerResearch
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Recommended Myeloma Expert – Professor Qiu Luguo – A Leading Specialist in Hematology and Multiple Myeloma
### Recommended Myeloma Expert – Professor Qiu Luguo – A Leading Specialist in Hematology and Multiple Myeloma

Expert
We at Advanced Medicine In China are proud to have Professor Qiu Luguo as our contracted expert. He provides telemedicine consultations for our international patients and can arrange for them to travel to China through a special fast-track system. Patients can receive treatment in the hospital where Professor Qiu works, under his direct supervision as their attending physician, ensuring comprehensive and professional care throughout the treatment process.
Professor Qiu is renowned in the field of hematologic oncology, particularly for treating patients who have not responded well to standard therapies. His deep expertise and innovative treatment approaches have brought new hope to patients, especially those with complex conditions. Through our services, international patients can not only access cutting-edge treatment recommendations via telemedicine but also come to China to receive the most advanced medical care.
The process is straightforward and efficient. Patients only need to submit the following documents, and our team will assist in arranging everything from teleconsultation to full treatment in China:
-
Pathology report
-
Latest discharge summary
-
Detailed description of the condition
-
History of treatment and its effectiveness
We recommend Professor Qiu for his exceptional achievements and extensive experience, which have provided a wide range of treatment options for countless hematologic cancer patients. His international reputation further affirms his outstanding contributions to the fields of hematology and multiple myeloma.
### Expert Profile
Professor Qiu Luguo is the Director of the Lymphoma Diagnosis and Treatment Center at the Hematology Hospital, Chinese Academy of Medical Sciences, and Director of the Tianjin Cord Blood Hematopoietic Stem Cell Bank. He has long been dedicated to both clinical and basic research in hematologic diseases. Professor Qiu is a recipient of the State Council’s special government allowance and has been recognized as an Outstanding Young and Middle-aged Expert by the National Health Commission of China. As a leading authority in lymphoma and multiple myeloma treatment, he is also a member of several international academic organizations, including the International Myeloma Society (IMS) and the International Myeloma Working Group (IMWG).
### Areas of Expertise
Professor Qiu is highly skilled in the diagnosis and treatment of lymphatic system tumors, particularly in the precise diagnosis, stratified treatment, and prognosis evaluation of lymphoma, chronic lymphocytic leukemia, and multiple myeloma. He has also made significant progress in the application of hematopoietic stem cell transplantation technology, offering new treatment options for patients with hematologic diseases.
### Research Contributions and International Recognition
Throughout his 30-plus-year career, Professor Qiu has completed more than 30 national research projects and published nearly 700 papers in leading academic journals, with over 160 of them in SCI-indexed journals. His research achievements have garnered widespread attention in China and have been highly regarded in the international hematology community. He has been invited to speak at numerous international conferences and actively promotes global academic collaboration and exchange.
### Major Research Directions
Professor Qiu’s research focuses on the pathogenesis and precision treatment strategies for lymphatic system tumors. His work spans a broad range of fields, from basic research to clinical applications, covering the diagnosis, prognosis, and personalized treatment of lymphoma, multiple myeloma, and chronic lymphocytic leukemia. Additionally, he has conducted in-depth studies on hematopoietic stem cell transplantation and stem cell engineering, striving to develop innovative therapies that improve patient cure rates.
### Publications and Scientific Achievements
Professor Qiu has authored five major medical books, holds five national invention patents, and has received multiple first-place awards for his scientific achievements in China. His groundbreaking work in the genomics of multiple myeloma and chronic lymphocytic leukemia has made significant progress in early diagnosis and targeted treatment of these diseases. His research has not only advanced the treatment of lymphatic system tumors but also set an example for young researchers in the field of hematology.
With his exceptional clinical and research accomplishments, Professor Qiu Luguo has become a key figure in the global hematology community. His contributions have not only brought hope to patients but also laid a solid foundation for future advancements in the treatment of blood diseases. Some of his major publications include:…
-
Genomic and transcriptomic profiling reveals distinct molecular subsets associated with outcomes in mantle cell lymphoma. J Clin Invest. 2022 Feb 1;132(3):e153283. (IF: 19.456)
-
Indirubin-3′-monoxime acts as proteasome inhibitor: Therapeutic application in multiple myeloma. E Bio Medicine. 2022 Apr;78:103950. (IF:11.205)
-
A Phase II Trial of the Bruton Tyrosine-Kinase Inhibitor Zanubrutinib (BGB-3111) in Patients with Relapsed/Refractory Waldenström Macroglobulinemia. Clin Cancer Res. 2021 Oct 15;27(20):5492-5501. (IF: 12.531)
-
A Phase I Study of a Novel Fully Human BCMA-Targeting CAR (CT103A) in Patients with Relapsed/Refractory Multiple Myeloma. Blood, 2021 May 27;137(21):2890-2901. (IF: 22.133)
-
High incidence of MYD88 and KMT2D mutations in Chinese with chronic lymphocytic leukemia. Leukemia. 2021 Aug;35(8):2412-2415. Jan 22. (IF:12.883)
-
YWHAE/14-3-3ε expression impacts the protein load contributing to proteasome inhibitor sensitivity in multiple myeloma. Blood. 2020,136(4):468-479. (IF:16.509)
-
Monitoring the cytogenetic architecture of minimal residual plasma cells indicates therapy-induced clonal selection in multiple myeloma. Leukemia. 2020,34(2):578-588. (IF:11.528)
-
Destabilizing NEK2 overcomes resistance to proteasome inhibition in multiple myeloma. J Clin Invest. 2018,128(7):2877-2893. (IF:12.87)
-
Epigenetic silencing of miR-137 induces drug resistance and chromosomal instability by targeting AURKA in multiple myeloma. Leukemia. 2017.31(5):123-1135. (IF:11.412)
-
Intratumoral genetic heterogeneity and number of cytogenetic aberrations provide additional prognostic significance in chronic lymphocytic leukemia. Genet Med.19(2):182-191. (IF:7.756)
### Online Consultation and Appointment Information
Professor Ke currently offers online video consultation services. Patients can make appointments through the following methods:
WhatsApp: Https://wa.me/+8613717959070
Wechat:doctors0152
Email: doctor.huang@globecancer.com
#HematologyExpert #MultipleMyeloma #LymphomaTreatment #BloodCancer #StemCellTransplantation #MedicalInnovation #Telemedicine #InternationalHealthcare #AdvancedMedicineInChina #PrecisionMedicine #ProfessorQiuLuguo #CancerResearch #GlobalHealth #PatientCare #Oncology
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China’s CAR-T Cell Therapy: Leading a New Revolution in the Treatment of Relapsed/Refractory Mantle Cell Lymphoma (MCL)

MCL
#CARTCellTherapy #MantleCellLymphoma #RelapsedMCL #RefractoryMCL #Hematology #MCL
Mantle Cell Lymphoma (MCL), a rare and complex subtype of non-Hodgkin’s lymphoma, exhibits both aggressive and indolent characteristics. Since MCL patients are usually diagnosed at an advanced stage, often with poor prognostic factors, traditional treatments such as chemotherapy and immunotherapy have brought survival benefits to some patients. However, most still face disease relapse or progression, particularly those with relapsed/refractory (r/r) MCL, where treatment poses significant challenges. For a long time, treatment options for MCL have been limited, making it difficult to achieve long-term remission or cure, highlighting the need for breakthroughs.
Chinese CAR-T Cell Therapy: Offering Hope for Relapsed/Refractory MCL With the rapid development of biotherapeutics, Chinese CAR-T cell therapy has emerged as a breakthrough innovation in lymphoma treatment. Since the approval of the world’s first CAR-T product in 2017, China has made significant progress in CAR-T research and application, especially in treating r/r MCL, where CAR-T therapy has shown excellent efficacy. CAR-T therapy not only significantly improves patient remission rates but also offers new survival opportunities for high-risk patients with poor prognoses.
Efficacy: In recent years, multiple international and Chinese clinical studies have confirmed the efficacy of CAR-T cell therapy in patients with relapsed/refractory MCL. Clinical data from China have also demonstrated outstanding results. In August 2024, the Chinese CAR-T product Relmacabtagene Autoleucel, a CD19-targeted CAR-T cell therapy, was approved for its third indication, treating adult patients with relapsed/refractory MCL (r/r MCL). Clinical data showed that Relmacabtagene Autoleucel achieved an overall response rate of 81.4%, with a complete response rate of 67.8%. The median progression-free survival (PFS) was 13 months, and the median overall survival (OS) was 19.5 months. These data further prove that Chinese CAR-T cell therapy not only has high efficacy in r/r MCL patients but also provides lasting survival benefits.
Side Effect Management: Despite its remarkable efficacy, CAR-T therapy is associated with certain side effects, such as cytokine release syndrome (CRS) and neurotoxicity (NT). However, with the continuous optimization of treatment technologies and accumulated experience in China, the incidence of side effects has been effectively controlled. In treating relapsed/refractory MCL patients, Chinese medical teams have been able to limit the occurrence of grade ≥3 CRS and NT to 6.8% in clinical practice. This lower toxicity makes CAR-T therapy safer in clinical applications, offering a new option for patients who cannot tolerate traditional treatments. Particularly for those who are resistant to Bruton’s tyrosine kinase inhibitors (BTKi) or ineligible for autologous hematopoietic stem cell transplantation, Chinese CAR-T therapy provides a gentler and effective treatment option.
Indications: The following groups of patients can benefit from Chinese CAR-T cell therapy:
-
Patients with relapsed/refractory MCL (r/r MCL)
-
MCL patients who do not respond to conventional treatments
-
Patients with poor prognostic factors (TP53 mutation, Ki67≥30%, blastoid MCL, pleomorphic MCL, POD24)
-
Patients previously exposed to BTKi or resistant/relapsed/refractory MCL
-
High-risk patients unsuitable for transplantation who cannot achieve good remission
Conclusion: With the widespread application of Chinese CAR-T therapy globally, more MCL patients, both domestic and international, are able to achieve deep remission and long-term survival. This not only marks a significant advancement in China’s hematological cancer treatment but also signifies that Chinese CAR-T cell therapy is leading a new transformation in the treatment of relapsed/refractory lymphoma worldwide.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CancerTreatment #Immunotherapy #CancerResearch #Chinesemedicalbreakthrough #LymphomaTreatment #Oncology #Biotherapy #CancerInnovation
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Meet Chinese Doctor. Xiaoyan Ke: Leading Expert in Hematology and Lymphoma Treatment
Meet Chinese Doctor. Xiaoyan Ke: Leading Expert in Hematology and Lymphoma Treatment

Global Healthcare
In the journey of treating hematologic malignancies, for patients in advanced stages with no standard clinical treatment options, seeking medical care abroad or through remote video consultations has become a pathway to new hope. Through these means, patients can connect with top international experts and gain access to new treatment plans and more options. Leading international specialists not only continuously achieve breakthroughs in treatment technologies but also provide personalized treatment recommendations based on the latest research. Next, we are proud to introduce a highly acclaimed and renowned expert in the fields of hematology and lymphoma from China—Professor Ke Xiaoyan.
### Expert Profile
**Professor Ke Xiaoyan** is the Chief Physician of the Hematology Department at Peking University Third Hospital and serves as the Lead Expert of the Adult Lymphoma Division at the GoBroad Medical (Hematology) Beijing Research Center. She is also the Deputy Director of the Hematology Department at Peking University Health Science Center. Additionally, she holds the position of Chief Consultant for the Adult Lymphoma Department at Beijing GoBroad-Boren Hospital, where she has trained numerous PhD students, establishing herself as one of China’s top experts in hematologic oncology.
Professor Ke is highly regarded within the Chinese medical community. She has served as the Chairperson of the Hematology Branch of the Chinese Female Physicians Association and currently holds key positions in several authoritative medical organizations. Her academic background and international reputation have earned her wide recognition in the field of hematologic malignancy treatment.
### Areas of Expertise
Professor Ke has extensive experience in the standardized treatment of lymphoma, targeted chemotherapy, and both autologous and allogeneic hematopoietic stem cell transplantation. She is at the forefront of international research in treating relapsed and refractory lymphomas through second-generation gene sequencing, targeted therapies, and CAR-T cell immunotherapy. Beyond her clinical expertise, she is a leading figure in the molecular diagnosis of malignant blood diseases and the monitoring of minimal residual disease.
### Research Contributions and International Reputation
Professor Ke has made remarkable achievements in scientific research as well. She has been the principal investigator in more than 70 international multicenter clinical trials and has published over 300 academic papers, including more than 30 influential SCI papers in top-tier international journals, focusing on cutting-edge research in lymphoma and hematologic malignancies. She has authored and contributed to more than 30 authoritative medical books and received over 40 national research grants, significantly advancing the field of hematology.
In addition to her research work, Professor Ke serves on the editorial boards of several medical journals, including as Deputy Editor-in-Chief of the **Journal of Leukemia & Lymphoma**. She is also a consulting expert for the Ministry of Health and a key member of the Central Health Consultation Committee. As a leading figure in international hematologic oncology research, her studies encompass the molecular mechanisms of lymphoma, immunotherapy, and stem cell transplantation, enhancing China’s standing in global academic research.
### Main Research Focuses
– Standardized treatment of lymphoma
– Molecular diagnosis of malignant blood diseases and monitoring of minimal residual disease
– Research on autologous peripheral blood stem cell transplantation for malignant blood diseases
– Non-myeloablative transplantation and CAR-T cell therapy for refractory relapsed malignant lymphoma
– Research on B7/CD28 signaling and T-cell immune tolerance mechanisms
– Study of the molecular mechanisms of tumor immunity
### Publications and Research Achievements
Professor Ke Xiaoyan has authored several authoritative books in the field of lymphoma, including **Lymphoma** and **Lymphoma Diagnosis and Treatment Manual**. Her key SCI papers include:
-
**Biological and Clinical Influences of NPM1 in AML Patients with DNMT3A Mutations**, *Cancer Management and Research*, 2018.
-
**Clinical and Biological Implications of IDH1/2 in AML with DNMT3A Mutation**, *Cancer Management and Research*, 2018.
-
**High PD-L1 Expression Predicts Poor Prognosis in Diffuse Large B-cell Lymphoma**, *Annals of Hematology*, 2018.
-
**Molecular Predictors of Post-transplant Survival in Acute Myeloid Leukemia**, *Blood Cancer Journal*, 2017.
-
**High Expression of CPNE3 Predicts Adverse Prognosis in AML**, *Cancer Science*, 2017.
-
**High Expression of ETS2 Predicts Poor Prognosis in AML**, *Journal of Translational Medicine*, 2017.
-
**A Minicircuitry of microRNA-9-1 and RUNX1-RUNX1T1 Contributes to Leukemogenesis in t(8;21) AML**, *International Journal of Cancer*, 2017.
### Online Consultation and Appointment Information
Professor Ke currently offers online video consultation services. Patients can make appointments through the following methods:
WhatsApp: Https://wa.me/+8613717959070
Wechat:doctors0152
Email: doctor.huang@globecancer.com
#Hematology #LymphomaTreatment #AdvancedCancerCare #InternationalConsultation #CAR_TTherapy #PersonalizedMedicine #ProfessorKeXiaoyan #StemCellTransplantation #CancerResearch #GlobalHealthcare
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Nature Medicine | New Breakthrough in China’s CAR-T Therapy: Significant Progress in Allogeneic CD5 CAR-T Treatment for Relapsed T-ALL
**Nature Medicine | New Breakthrough in China’s CAR-T Therapy: Significant Progress in Allogeneic CD5 CAR-T Treatment for Relapsed T-ALL**

T-ALL
#CAR-TTherapy #TALL #ALL #Leukemia #RelapsedLeukemia #TCellLeukemia #CD5 #CD7 #CART
Recently, a collaborative research study conducted by multiple Chinese medical and research institutions was published in *Nature Medicine*. This study, titled “Allogeneic CD5-specific CAR-T Therapy for Relapsed/Refractory T-ALL: A Phase 1 Trial,” demonstrated the potential of CD5 CAR-T cells in treating relapsed/refractory acute T-cell lymphoblastic leukemia (R/R T-ALL).
### Innovative Therapy Brings Hope
For a long time, patients with relapsed/refractory T-ALL have lacked effective treatment options and faced a poor prognosis. In recent years, CD7 CAR-T cell therapy has shown some efficacy in these patients, but CD7-negative relapse remains a significant challenge. This study focuses on evaluating the safety, efficacy, and pharmacokinetics of CD5 CAR-T cells, offering new insights for future treatments.
The study included 19 patients, most of whom had previously received CD7 CAR-T therapy. Results showed that this therapy was safe, with no dose-limiting toxicity observed, and adverse events were primarily manageable hematologic toxicity. In terms of efficacy, all patients achieved complete remission within 30 days of treatment. The study also demonstrated, for the first time, the persistence of CD5 CAR-T cells in the body and their ability to eliminate CD5+ T cells, indicating strong anti-tumor activity.
### Breakthrough in Treating Relapsed Patients
In addition to safety and efficacy, the research team explored the coexistence of CD7 CAR-T and CD5 CAR-T cells and studied immune cell changes in the patients. For those who relapsed after CD7 CAR-T treatment with CD7-negative cells, CD5 CAR-T offered a new salvage therapy, providing additional treatment options for such patients.
### Multidisciplinary Collaboration Drives Clinical Progress
Unlike CAR-T therapies for B-cell tumors, treating T-cell malignancies poses more challenges, particularly in controlling immune deficiencies. Dr. Jing Pan and her team conducted in-depth research on target selection and relapse mechanisms, while also focusing on balancing treatment safety and efficacy.
This study was made possible by the collaborative efforts of various medical institutions in China, collectively opening up a new therapeutic pathway for T-cell tumor patients.
### Looking Ahead
As innovations and advances in T-ALL treatment continue, the team plans to further research CD5 CAR-T therapy and collaborate with experts across various fields to optimize CAR-T treatment protocols, helping more patients with T-cell lymphoblastic tumors overcome their diseases.
This series of research achievements not only brings new hope to patients with R/R T-ALL but also provides valuable insights for the future development and optimization of CAR-T therapies. The team remains committed to a patient-centered approach, striving to drive continuous breakthroughs and innovations in T-cell tumor treatment.
To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#Immunotherapy #CancerTreatment #TALLResearch #LeukemiaBreakthrough #Hematology #CellTherapy #CancerInnovation #CancerResearch #ClinicalTrials #MedicalBreakthrough
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese Medical Team: Long Survival and Significant Benefits with BCMA CAR-T Treatment for RRMM: A 5-Year Outlook
**Chinese Medical Team: Long Survival and Significant Benefits with BCMA CAR-T Treatment for RRMM: A 5-Year Outlook**

RRMM
Chimeric antigen receptor (CAR) T-cell therapy is one of the most promising new treatments for relapsed/refractory multiple myeloma (RRMM), but reports on its long-term efficacy and safety are limited. As early as 2022, Professor Du Juan’s team from the Department of Hematology at Shanghai Changzheng Hospital published a Phase I/II study demonstrating that patients with poor physical status could also benefit from CAR-T therapy. Recently, the team updated their findings with a five-year long-term follow-up, focusing on factors affecting long-term clinical benefits. The results were published in *Clinical Cancer Research*. The following summary of the study’s content is provided by *Cancer Information* for readers’ benefit.
### Evidence for Long-Term Efficacy and Safety of BCMA CAR-T Cell Therapy
#### Patient Characteristics
The study included 49 RRMM patients who had all received at least three prior lines of therapy before undergoing BCMA CAR-T cell treatment. At enrollment, 20 patients (40.82%) had poor physical status (ECOG performance status of 3-4), 42.86% had high-risk cytogenetic features, and 63.27% had received four or more lines of treatment. At the time of infusion, 79.59% had progressive disease. Among the patients with poor physical status, 30% had extramedullary disease (EMD), 45% had high-risk cytogenetic features, 70% had received four or more lines of treatment, and 80% had progressive disease after their last line of treatment.
#### Efficacy Evaluation of BCMA CAR-T Cell Therapy HDS269B
After a median follow-up of 59.0 months, the study showed an overall response rate (ORR) of 77.55%. The ORR was similar across patients with different ECOG scores. The median progression-free survival (PFS) was 9.5 months, and the median overall survival (OS) was 20.0 months. The five-year PFS and OS rates were 21.3% and 31.4%, respectively. For patients with ECOG scores of 0-2, the median PFS was 11.0 months, compared to 4.0 months for those with scores of 3-4 (P=0.18). The median OS was 41.8 months for ECOG 0-2 patients and 10.5 months for ECOG 3-4 patients (P=0.015).
Patients who had previously undergone four or more lines of therapy had significantly shorter PFS and OS compared to those who had received fewer than four lines (PFS: P=0.012; OS: P=0.0049). Among the 11 patients with EMD at enrollment, the ORR was 64% for those with EMD and 82% for those without EMD. However, median PFS and OS were notably shorter in patients with EMD (PFS: 3.0 months vs. 10.5 months, P=0.06; OS: 5.0 months vs. 24.0 months, P=0.03).
#### MRD-Negative Status and CAR-T Cell Persistence Indicate Better Long-Term Survival
Minimal residual disease (MRD) negativity was significantly associated with longer PFS and OS. In this study, MRD data were available for 22 patients on day 28 post-infusion, with 14 patients (63.64%) achieving MRD negativity (10^-4). These patients experienced significantly longer PFS and OS compared to MRD-positive patients. Similar associations were observed with MRD status at 3 and 6 months post-infusion.
The expansion of CAR-T cells was also closely related to clinical outcomes. Patients who achieved partial response (PR) or better had higher CAR-T cell peak levels. Patients without disease progression five years post-infusion had significantly higher CAR-T cell expansion peaks than those with progression. Additionally, the duration of CAR-T cell persistence correlated with longer PFS and OS, with patients having CAR-T cells persisting for ≥6 months, ≥12 months, ≥24 months, and ≥36 months showing significantly better PFS and OS than those without detectable CAR-T cells.
#### Controlled Safety Profile of BCMA CAR-T Cell Therapy HDS269B
No new CAR-T cell-related toxicities were observed during long-term follow-up. All patients experienced at least one adverse event (AE), with the most common long-term (≥28 days post-infusion) grade ≥3 AEs being hematologic in nature. No second primary malignancies or delayed immune effector cell-associated neurotoxicity syndrome (ICANS) were observed.
This study also included survival analysis, classifying patients by PFS and OS. The results indicated that ECOG 0-2 status, fewer than four prior therapies, and CAR-T cell persistence for ≥6 months were independently associated with longer survival.
### The Potential of BCMA CAR-T Therapy and the Need for Future Optimization
Through a five-year long-term follow-up of 49 RRMM patients, this study systematically evaluated the efficacy and safety of BCMA CAR-T cell therapy HDS269B. The findings suggest that poor physical status is not a contraindication for CAR-T therapy, thus broadening the indications for this treatment. While the results are encouraging, the study has some limitations, including its open-label, single-arm design and small sample size, which, combined with the long follow-up period, could lead to some patient attrition. Furthermore, despite the lack of new severe toxicities, long-term safety requires continued observation.
Overall, this study underscores the importance of BCMA CAR-T cell therapy in the treatment of RRMM and provides a crucial basis for exploring and applying CAR-T immunotherapy in the frontline treatment of multiple myeloma.

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +86137 1795 9070
Email: doctor.huang@globecancer.com
#CAR_Therapy #BCMACART #MultipleMyeloma #CancerResearch #LongTermSurvival #MedicalInnovation #Hematology #CancerTreatment #Immunotherapy #OncologyAdvances
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Expert Perspective: Side Effects and Management of CAR-T Therapy for a Thai Patient
**Expert Perspective: Side Effects and Management of CAR-T Therapy for a Thai Patient**
At the Hematology Department of Shanghai Tongji Hospital, Dr. Li Ping, the chief physician for the elderly Thai patient Ms. P, provided a detailed overview of the patient’s multiple myeloma condition and treatment journey. After experiencing multiple treatments and relapses in Thailand, the patient ultimately chose and trusted CAR-T therapy in China. Dr. Li highlighted that the most common side effect is cytokine release syndrome (CRS), which manifests as fever, hypotension, and difficulty breathing. While most CRS cases are mild to moderate, severe CRS can be life-threatening. She also emphasized that through scientific management, the team’s extensive experience, and the low immunogenicity of the fully human CAR-T product FUCASO, the side effects of CAR-T therapy can be effectively controlled, offering the patient hope for a cure.
We will continue to follow up on this patient’s treatment progress and provide updates.
#CARTherapy #MultipleMyeloma #FUCASO #Equecel #TongjiHospital #Shanghai #MedicalInnovation #CancerTreatment #Hematology #PatientJourney #Immunotherapy
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
High Risk Multiple Myeloma Solution and Chinese Expert Consensus
**High Risk Multiple Myeloma Solution and Chinese Expert Consensus**

Multiple Myeloma
High risk multiple myeloma (HRMM) refers to patients with multiple myeloma whose overall survival is less than 2 to 3 years under current standard treatments.
In 2024, the Chinese Society of Clinical Oncology’s Multiple Myeloma Expert Committee and the Chinese Anti-Cancer Association’s Hematologic Oncology Committee, organized by relevant experts, developed the “Chinese Expert Consensus on the Diagnosis and Treatment of High Risk Multiple Myeloma (2024 Edition),” which was officially published in the *Chinese Journal of Hematology* in May 2024. This consensus defines HRMM, outlines high-risk factors and risk stratification systems, and provides key treatment recommendations for HRMM, aiming to improve the quality of life and prognosis for HRMM patients in China.
**Definition of HRMM**
There is currently no precise definition of HRMM. Referencing the International Myeloma Working Group (IMWG)’s definition, the Chinese Expert Committee considers HRMM patients as those with an overall survival (OS) of less than 3 years after receiving autologous hematopoietic stem cell transplantation (auto-HSCT) or less than 2 years if they have not received auto-HSCT. Patients with OS of less than 2 years after receiving auto-HSCT are classified as ultra-high risk multiple myeloma (UHRMM) patients.
**Prognostic Factors of HRMM**
The biological characteristics of MM tumor cells and treatment response are key determinants in identifying HRMM.
**Static Prognostic Factors of MM**
-
**Genetic High-Risk Factors:**
In the context of genetic high-risk factors, cytogenetic abnormalities are core indicators in the MM risk stratification system, but there is still some debate over the definition of high-risk cytogenetic abnormalities (HRCAs). The “National Comprehensive Cancer Network (NCCN) Guidelines (2024.v1)” indicate that the presence of multiple HRCAs correlates with a poorer prognosis. Fluorescence in situ hybridization (FISH) is currently the main genetic testing technique for MM, and next-generation sequencing can be performed if conditions allow (TP53 mutations have a significant impact on prognosis, while the effects of KRAS, NRAS, DIS3, BRAF, and FAM46C are less clear). All these genetic tests require enrichment and selection of plasma cells.
-
**Non-Genetic High-Risk Factors:**
Confirmed non-genetic prognostic factors include International Staging System (ISS) stage III, extramedullary disease excluding bone lesions, circulating plasma cells, high plasma cell proliferation index, elevated lactate dehydrogenase (LDH), frailty, renal insufficiency, and thrombocytopenia.
**Dynamic Prognostic Factors of MM**
-
**Duration of Initial Treatment Response:**
The duration of response to initial treatment is a crucial dynamic prognostic factor for MM. Patients who received auto-HSCT followed by maintenance therapy and experienced relapse/progression within less than 2 years are classified as HRMM; for those who did not receive auto-HSCT, relapse within less than 18 months after starting treatment also indicates HRMM. Functional high risk refers to MM patients without known genetic high-risk factors at diagnosis who experience early progression within 18 months after the start of treatment.
-
**Depth of Initial Treatment Response:**
The depth of response to initial treatment is another important dynamic prognostic factor for MM. Patients who achieve negativity for minimal residual disease (MRD) in both bone marrow and imaging studies have the best survival outcomes. Achieving MRD negativity can partially overcome the adverse prognosis associated with high-risk cytogenetics. Continuous dynamic MRD monitoring has greater clinical value than a single MRD result, as sustained MRD negativity for more than 12 months can translate into long-term survival.
The Expert Committee considers newly diagnosed MM to be classified as HRMM if any of the following criteria are met:
-
R-ISS stage III, extramedullary disease excluding bone lesions, presence of circulating plasma cells (plasma cell leukemia is defined as ≥5% plasma cells in peripheral blood), presence of one or more HRCAs [t(4;14), t(14;16), t(14;20), del(17/17p), 1q21 gain/amplification, del(1p32), TP53 mutation], although 1q21 gain alone does not define HRMM;
-
MM patients who have received auto-HSCT followed by maintenance therapy and experience relapse within less than 2 years from the start of treatment;
-
Patients who have not received auto-HSCT and experience relapse within less than 18 months from the start of treatment;
-
Functional high risk;
-
Extramedullary relapse/secondary plasma cell leukemia;
-
New occurrence of 1q21 gain/amplification and/or del(17/17p)/TP53 mutation at relapse.
**Treatment of HRMM**
**Principles of Treatment for Newly Diagnosed HRMM**
The standard treatment for HRMM has not yet been established. The overall treatment strategy includes:
-
Utilizing combination therapies with drugs that have different mechanisms of action;
-
Aiming to eradicate all tumor clones, with the goal of achieving and maintaining MRD negativity both inside and outside the bone marrow;
-
Implementing a treatment strategy that adjusts based on the effectiveness of the therapy;
-
Acknowledging that current treatment outcomes for HRMM are still unsatisfactory, and encouraging the exploration of experimental therapies.
**Treatment for Newly Diagnosed HRMM Suitable for Transplantation**
-
**Induction Therapy Before Transplantation:**
For HRMM, induction therapy with the RVd regimen as a bridge to auto-HSCT has not met expectations in terms of depth of response and long-term prognosis, and achieving MRD negativity is more challenging compared to standard-risk patients. Some studies with novel drug-modified regimens have shown that patients with one HRCA receiving the KRd regimen (carfilzomib, lenalidomide, and dexamethasone) sequentially followed by auto-HSCT achieved similar MRD negativity rates and progression-free survival (PFS) as standard-risk patients, with no statistically significant difference. Meta-analyses indicate that incorporating a CD38 monoclonal antibody as part of the treatment backbone in early-line therapy provides clinical benefits for patients with HRCAs. For UHRMM patients, more intensive treatment regimens, such as the Dara-VRdC regimen (OPTIMUM/MUKnine study) and the Dara+KTD-PACE regimen (TT7 study), can be considered.
-
**Auto-HSCT:**
Tandem transplantation involves performing a planned second auto-HSCT within 3–6 months after the first. It is recommended that HRMM patients collect sufficient hematopoietic stem cells for two auto-HSCTs during the first mobilization. Regardless of the response achieved after the first transplant, it is advised to perform the tandem transplant within six months. The conditioning regimen for both transplants typically includes high-dose melphalan.
-
**Consolidation Therapy:**
If tandem transplantation is not performed, the original induction regimen can be continued for consolidation therapy for an additional 2–4 cycles.
-
**Maintenance Therapy:**
For HRMM patients, maintenance therapy should consider a combination of proteasome inhibitors, immunomodulators, and CD38 monoclonal antibodies, in either dual or triple drug regimens. It is recommended to continue maintenance therapy until disease progression or intolerance.
-
**Allo-HSCT:**
The long-term efficacy of allo-HSCT remains debatable and should only be considered within the context of clinical trials and for select high-risk patients.
#HRMM #AutoHSCT #CancerTreatment #MultipleMyeloma #InductionTherapy #TandemTransplantation #ConsolidationTherapy #MaintenanceTherapy #ProteasomeInhibitors #Immunomodulators #CD38MonoclonalAntibodies #AlloHSCT #UHRMM #NovelTherapies
Expert Consensus on the Treatment of HRMM
①Induction Therapy: For pre-transplantation induction therapy in HRMM, it is recommended to use a regimen based on CD38 monoclonal antibodies combined with proteasome inhibitors and immunomodulators. Recommended regimens include: Dara+KRd, Isa+KRd, Dara+VRd, and Isa+VRd. For patients who cannot tolerate a four-drug regimen, the KRd regimen is an alternative. For patients with significant extramedullary involvement (soft tissue or peripheral blood), additional cytotoxic drugs and, if necessary, radiotherapy can be added.
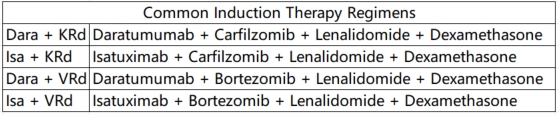
Therapy Regimens
②Auto-HSCT: Early auto-HSCT is the standard treatment for HRMM. For patients who receive auto-HSCT without significant adverse effects, tandem transplantation within six months post-transplant is recommended.
③Consolidation Therapy: For patients who do not undergo tandem transplantation, it is advised to continue consolidation therapy with the original induction regimen for 2–4 cycles.
④Maintenance Therapy: Maintenance therapy should involve a combination of proteasome inhibitors, immunomodulators, and CD38 monoclonal antibodies, either as dual or triple drug regimens. Therapy should continue until disease progression or intolerance.
⑤Clinical Research: Clinical studies targeting HRMM are encouraged, and it is recommended that HRMM patients prioritize enrollment in clinical trials.
**Treatment for Newly Diagnosed HRMM Not Suitable for Transplantation**
For MM patients not suitable for transplantation, an individualized treatment plan should be selected based on the patient’s fitness status score (IMWG GA score is recommended). For patients with good or moderate health, it is recommended to continue using the same regimen as for transplant-eligible patients. For frail patients, the VRd-lite (modified bortezomib + lenalidomide + dexamethasone) regimen and the DRd (daratumumab + lenalidomide + dexamethasone) regimen are currently the most commonly used first-line treatments.
**Treatment of Relapsed HRMM**
For patients with functional high risk and those defined as HRMM based on dynamic risk factors, re-induction regimens should include combinations of next-generation drugs or drugs with different mechanisms of action. Several clinical studies have shown that CAR-T cell (BCMA CAR-T) therapy can sustain efficacy in relapsed HRMM.
**Expert Consensus**
-
For relapsed HRMM patients, it is recommended to select combination regimens involving next-generation drugs or drugs with different mechanisms of action.
-
Patients with relapsed HRMM are encouraged to participate in clinical studies of CAR-T cell therapy or bispecific antibody immunotherapy.
**Summary**
For the treatment of HRMM, it is recommended to use multi-drug combination therapies with different mechanisms of action and bridge to auto-HSCT, aiming for deep and sustained MRD negativity and prolonging overall survival (OS) in patients. Currently, clinical trials of CAR-T cell therapy for newly diagnosed HRMM are being conducted, and combining auto-HSCT with CAR-T cell therapy can leverage the therapeutic benefits of both. Besides the BCMA target, CAR-T cell therapies targeting GPRC5D and FcRH5, as well as bispecific antibodies, have shown good efficacy in relapsed and refractory MM. New drugs and innovative diagnostic and therapeutic strategies, including immunotherapy, hold promise for overcoming the challenges in HRMM treatment.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#HighRiskMultipleMyeloma #HRMM #MultipleMyeloma #CancerTreatment #Hematology #Oncology #MedicalResearch #CancerCare #CancerAwareness #ChinaMedicalAdvances #AutoHSCT #MedicalConsensus #UHRMM #BloodCancer
#CancerPrognosis #GeneticRiskFactors #CancerResearch #MMTreatment #MRD #Hematology #CancerSurvival #PrognosticFactors
#CancerCriteria #Hematology #HighRiskMyeloma #PlasmaCellLeukemia #CancerRelapse #CytogeneticAbnormalities
#InductionTherapy #TandemTransplantation #ConsolidationTherapy #MaintenanceTherapy #ProteasomeInhibitors #Immunomodulators #CD38MonoclonalAntibodies #AlloHSCT #NovelTherapies
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

2024EHA | Professor Huang He: Targeting CD7 Universal CAR-T Therapy Shows Significant Efficacy and Good Safety in Treating R/R CD7+ T-ALL/LBL Patients
**2024EHA | Professor Huang He: Targeting CD7 Universal CAR-T Therapy Shows Significant Efficacy and Good Safety in Treating R/R CD7+ T-ALL/LBL Patients**
The 29th Annual Meeting of the European Hematology Association (EHA) 2024 concluded successfully on June 16, 2024, in Madrid, Spain. As a premier event in the international hematology field, it showcased the latest advancements in foundational and clinical practices.
At this year’s EHA meeting, several studies from Professor Huang He’s team at the First Affiliated Hospital, Zhejiang University School of Medicine were selected. One of these studies, titled “Phase I Study of Targeting CD7 Universal CAR-T Therapy for Relapsed/Refractory CD7+ T-ALL/LBL Patients,” was featured in a poster presentation.
T-cell acute lymphoblastic leukemia (T-ALL) and T-cell lymphoblastic lymphoma (T-LBL) are aggressive malignancies originating from precursor T cells, often affecting the bone marrow (BM), central nervous system (CNS), and mediastinum. Despite multiple lines of chemotherapy or allogeneic hematopoietic stem cell transplantation, some patients progress to a relapsed/refractory stage with poor prognosis, posing a significant clinical challenge. Current CAR-T cell therapies primarily target B-cell hematologic malignancies and multiple myeloma, such as CD19 CAR-T and BCMA CAR-T, while mature targets for T-cell hematologic malignancies are lacking. Preliminary research data indicate that CD7 could be a potential therapeutic target for T-cell hematologic malignancies. Based on this, the team developed a universal CAR-T product targeting CD7, named RD13-02, characterized by CRISPR/Cas9 technology to modify T-cell receptors (TCR) and CD7, creating an innovative “off-the-shelf” CAR-T cell therapy product aimed at truly achieving shelf-ready cell therapy.
The objective of this study was to evaluate the safety and efficacy of RD13-02 in treating R/R T-ALL/LBL. A total of 12 eligible R/R T-ALL/LBL patients (7 ALL, 5 LBL) were enrolled in the study, all exhibiting CD7 antigen expression. Six patients had extramedullary disease; all had blast cells in the bone marrow, with a median proportion of 64% (range: 6% to 89%), indicating high-risk end-stage patients. The patients, aged 3 to 70 years, were enrolled in a “3+3” dose-escalation study. Based on the total dose of RD13-02, they were randomly divided into four dosage groups (DL-1: 0.5×10^8 CAR+T cells; DL1: 2×10^8 CAR+T cells; DL2: 4×10^8 CAR+T cells; DL3: 6×10^8 CAR+T cells). All patients received a standardized lymphodepletion regimen of fludarabine (30mg/m2/day) and cyclophosphamide (500mg/m2/day) for three consecutive days (Day -5 to Day -3) before CAR-T infusion. Preliminary disease assessment was conducted on Day 28. After determining the MTD and RP2D, dose expansion will be carried out.
The study results indicated that no dose-limiting toxicity (DLT), immune effector cell-associated neurotoxicity syndrome (ICANS), or graft-versus-host disease (GVHD) was observed at any dose level. Cytokine release syndrome (CRS) was manageable, mostly mild (G1, n=8; G2, n=2; G3, n=2). Due to the occurrence of Grade 3 CRS in one patient each in the DL2 and DL3 groups, the DL1 dose was set as RP2D, focusing on safety and efficacy. In the initial 28-day evaluation post-infusion, 9 out of 12 patients achieved CR/CRi. By the second month post-infusion, an additional 2 patients achieved CR/CRi, with an ORR of 92%. All patients showed expansion observed through qPCR, with the expansion occurring between Days 6 to 15 and peaking between Days 8 to 27, with a median duration of 33 days (15-79). The study preliminarily confirmed that the anti-CD7 universal CAR-T product RD13-02 is safe and effective for CD7+ R/R T-ALL/LBL. Long-term efficacy needs more patients and extended follow-up for validation. To date, this study has shown the best clinical results for universal CAR-T therapy for R/R T-ALL/LBL, attracting widespread attention from peers both domestically and internationally at this EHA meeting. Additionally, the Bone Marrow Transplantation Center at the First Affiliated Hospital, Zhejiang University School of Medicine is developing multiple targeted CAR-T cell therapy products for various hematologic malignancies, including B-cell, T-cell, and myeloid malignancies.
**Current Clinical Treatment of T-ALL/LBL**
The treatment of T-ALL/LBL remains a significant clinical challenge. Current strategies involve achieving remission with existing frontline therapies followed by sequential allogeneic or autologous hematopoietic stem cell transplantation, with most patients achieving long-term survival. However, some patients face refractory or relapsed disease with poor prognosis. For these patients, clinical studies of new drugs or treatment strategies, such as innovative drugs based on disease targets and pathways, are actively being conducted but have not achieved significant progress. CAR-T cell therapy, with its excellent efficacy and safety, is currently the most promising innovative therapy. It is hoped that with further research, CAR-T cell therapy can bring more survival benefits to T-ALL/LBL patients.
**Challenges and Strategies in CAR-T Cell Therapy**
Cellular immunotherapy has been a milestone in the medical field over the past decade, showing breakthrough efficacy in hematologic malignancies, but it still faces several challenges that need to be addressed.
First, the discovery of new targets. Current mature CAR-T cell therapy products mainly focus on B-cell hematologic malignancies, and new targets are urgently needed for T-cell, myeloid, and solid tumors.
Second, improving efficacy. Existing CAR-T cell therapy for B-cell hematologic malignancies has an efficacy rate of about 50%, and continuous technological improvements are expected to develop more effective cell therapy products for clinical application.
Third, existing CAR-T products are autologous CAR-T, which are costly and have long treatment cycles, necessitating the development of universal CAR-T to improve patient compliance.
The team’s novel cell therapy research, including the study selected for this EHA meeting, addresses these clinical challenges. Additionally, last year, the team published results on “function-enhanced” CAR-T in the journal *Nature*, using PD-1 site-specific integration and non-viral delivery methods to successfully prepare CAR-T cells, significantly enhancing their therapeutic effects.
Furthermore, managing CAR-T cell therapy complications, such as ICANS and CRS, requires in-depth exploration. Progress has been made in understanding the mechanisms of complications, leading to gene functionalization of CAR-T cells and developing “smart” CAR-T products to achieve “efficacy enhancement and toxicity reduction.” Additionally, exploring CAR-T combinations with small molecule targeted drugs and immunotherapeutic drugs could achieve better efficacy. The team recently published in the *New England Journal of Medicine* on the sequential “integrated” regimen of CD7 CAR-T cell therapy and allogeneic hematopoietic stem cell transplantation (HSCT), showing excellent efficacy in relapsed/refractory hematologic malignancies.
**Future Prospects of Cellular Therapy**
It is well known that hematology has always been at the forefront of medical development, including the application of the first small molecule targeted drugs and the first antibodies. The remarkable efficacy of cellular therapy in hematologic diseases has demonstrated its great potential in clinical practice. In the future, it is believed that with the development and commercialization of more novel cellular immunotherapy products, their efficacy will continue to improve, side effects will gradually decrease, and indications will expand.
In the field of hematologic diseases, in addition to traditional chemotherapy, small molecule targeted drugs, antibody drugs, and hematopoietic stem cell transplantation, cellular therapy has improved the clinical treatment landscape of hematologic diseases and holds a significant position. It is anticipated that in the future, the treatment of hematologic diseases may enter a chemotherapy-free era, bringing higher cure rates and better quality of life for patients.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#CAR_T #TALL #LBL #EHA2024 #Immunotherapy #Hematology #CancerResearch #CD7 #CellTherapy #MedicalAdvancements
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

2024 EHA | New Generation BCL-2 Inhibitor Sonrotoclax Expected to Be a New Treatment Option for RRMM
**2024 EHA | New Generation BCL-2 Inhibitor Sonrotoclax Expected to Be a New Treatment Option for RRMM**

RRMM
The 29th Annual Meeting of the European Hematology Association (EHA) was held from June 13-16, 2024, in Madrid, Spain. During this event, the results of the study on the new-generation BCL-2 inhibitor Sonrotoclax (BGB-11417) combined with dexamethasone for the treatment of relapsed/refractory multiple myeloma (RRMM) patients with t(11;14) were presented.
**Ib/II Phase Study of Sonrotoclax (BGB-11417) Combined with Dexamethasone for the Treatment of RRMM with t(11;14)**
**Study Background**
The B-cell lymphoma-2 (BCL-2) protein helps multiple myeloma (MM) tumor cells evade apoptosis and promote cell survival. Studies have found that MM cells with t(11;14) have significantly higher BCL-2 protein expression compared to other cells. BCL-2 inhibitors can block anti-apoptotic mechanisms and induce cell apoptosis. Currently, data have shown that BCL-2 inhibitors have significant anti-MM potential in RRMM patients with t(11;14) who have failed multiple lines of treatment. However, to date, no BCL-2 targeted therapy has been approved for MM treatment.
Sonrotoclax (BGB-11417) is a new-generation BCL-2 inhibitor. Preclinical studies have found that it has over ten times the BCL-2 inhibitory ability of the first-generation BCL-2 inhibitors, a shorter half-life, and no dose accumulation. BGB-11417-105 (NCT04973605) is an ongoing Ib/II phase trial aimed at evaluating the efficacy and safety of Sonrotoclax combined with dexamethasone ± carfilzomib/daratumumab/pomalidomide in treating RRMM patients with t(11;14). The latest data from this study were reported at this EHA meeting.
**Study Design**
The enrolled patients were all RRMM with t(11;14), who had failed at least three treatments, including proteasome inhibitors (PI), immunomodulatory drugs (IMIDs), and anti-CD38 monoclonal antibodies, and were refractory/relapsed to the most recent treatment. In the first part, dose escalation cohorts, patients took 80, 160, 320, or 640 mg of Sonrotoclax daily, combined with 40 mg of dexamethasone weekly until intolerance, disease progression, or death. The primary endpoints were safety/tolerability, determining the maximum tolerated dose (MTD)/maximum assessed dose (MAD), and recommending the dose for the expansion phase (RDFE). The second part, dose expansion, primarily evaluated the tolerability and antitumor activity of Sonrotoclax combined with dexamethasone ± carfilzomib/daratumumab/pomalidomide.
**Study Results**
The initial report at 2023 ASH showed that Sonrotoclax (640 mg) was well tolerated, with no dose-limiting toxicities (DLTs) observed in any patients, establishing 640 mg as the RDFE. The overall response rate (ORR) for this cohort was 70%, and the rate of very good partial response (≥VGPR) was 40%.
Updated in this report: as of March 25, 2024, 32 patients received the RDFE dose of 640 mg Sonrotoclax combined with dexamethasone (10 in the first part and 22 in the second part). The median follow-up time was 4.6 months (0.1-19 months).
The median age of patients was 69 years (48-80 years), with a median of 3 prior lines of treatment (1-12 lines), and 28.1% had high-risk cytogenetic abnormalities. All patients had been exposed to PI and IMiD treatments, and 72% had been exposed to anti-CD38 monoclonal antibody treatments. 56% were PI-refractory, 72% were IMiD-refractory, 56% were anti-CD38 monoclonal antibody-refractory, and 47% were triple-refractory.
**Safety**
No patients experienced DLTs. The most common treatment-emergent adverse events (TEAEs) were fatigue and insomnia (28% each), diarrhea (22%), constipation, and nausea (16% each). Only 4 patients (13%) experienced hematologic TEAEs (grade 3 thrombocytopenia, grade 1 and 3 platelet count decrease, and grade 3 neutropenia). Two patients died, both unrelated to treatment (one due to pancreatic cancer complications and one due to liver cancer and liver failure).
**Efficacy**
Among the 24 evaluable patients, the ORR was 75%, with a ≥VGPR rate of 50%. The complete response (≥CR) rate was 21% (CR, n=4; sCR, n=1), with two patients achieving minimal residual disease (MRD) negativity (10^-5). The median time to response was 0.7 months, and the median duration of response (DOR) was 8 months. As of the follow-up, the longest DOR was 18 months.
**Conclusion**
Sonrotoclax (640 mg) combined with dexamethasone was well tolerated in heavily pretreated RRMM patients with t(11;14), with low rates of hematologic toxicity and infection. It provided deep and durable responses: ORR was 75%, ≥VGPR rate was 50%, and ≥CR rate was 21%. This study will continue to explore Sonrotoclax in combination with other novel agents.
**Interpretation by Professor Lu Jin**
The t(11;14) translocation is a common genetic abnormality in MM patients, present in approximately 15%-20% of newly diagnosed MM cases. Before the era of novel drugs, many researchers believed that patients with t(11;14) had favorable treatment outcomes and were classified as a standard-risk group. However, recent studies have found that patients with t(11;14) are less sensitive to bortezomib regimens, and even with the VRd regimen, patients with t(11;14) have significantly lower deep response rates (≥VGPR) and PFS benefits compared to other standard-risk patients. This suggests that t(11;14) may be an influencing factor for poor efficacy of novel drugs, necessitating other therapies to improve prognosis.
Studies have found that tumor cells with t(11;14) often have high BCL-2 expression and high sensitivity to BCL-2 inhibitors. The phase III CANOVA study demonstrated that venetoclax combined with dexamethasone resulted in deeper response rates (ORR 62% vs. 35%, p<0.001; ≥VGPR rate 39% vs. 14%, p<0.001) and longer median PFS (9.1 months vs. 4.9 months, p=0.237) compared to pomalidomide combined with dexamethasone in treating RRMM patients with t(11;14), though without significant statistical difference. The failure to meet the primary endpoint might be due to more patients in the control group not reaching IMWG-defined disease progression and thus being treated with new regimens, resulting in censored data in the initial PFS analysis. In the latest post-hoc analysis, including new treatment as an event in PFS, the analysis showed a significant statistical difference in median PFS (9.4 months vs. 4.0 months, p=0.003).
Therefore, the benefits of BCL-2 inhibitors still warrant further exploration. Sonrotoclax is a second-generation highly selective and potent BCL-2 inhibitor. In preclinical trials, Sonrotoclax had an IC50 for BCL-2 protein over ten times lower than that of the first-generation BCL-2 inhibitors (0.014 nM vs. 0.2 nM). It also showed ≥2000 times selectivity over BCL-XL, BCL-W, MCL-1, and BCL2A1 and stronger cytotoxicity against MM cell lines. Additionally, Sonrotoclax has a shorter half-life (approximately 4.5 hours), allowing more flexible exploration of rapid dose escalation plans without the risk of off-target toxicity due to drug accumulation.
The Ib/II phase study reported at this EHA meeting demonstrated that Sonrotoclax combined with dexamethasone was well tolerated in RRMM patients previously treated with multiple lines of therapy. The 640 mg RDFE dose achieved a 75% ORR, ≥VGPR rate of 50%, and ≥CR rate of 21%. We look forward to the release of more results from Sonrotoclax combination therapies. Additionally, as the proportion of t(11;14) in systemic light chain amyloidosis and plasma cell leukemia is higher than in MM, the exploration of BCL-2 inhibitors in these plasma cell diseases is also ongoing.
**Professor Lu Jin**
Chief Physician, Professor, Ph.D. Supervisor
Peking University People’s Hospital, Peking University Institute of Hematology
Specializes in clinical and laboratory research on multiple myeloma, primary systemic amyloidosis, lymphoma, and cellular immunotherapy.
General Secretary and Standing Committee Member of the Hematology Physician Branch of the Chinese Medical Doctor Association
President of the Hematology Physician Branch of the Beijing Medical Doctor Association
Vice Chairman of the Multiple Myeloma Professional Committee and the Histiocyte Disease Professional Committee of the Chinese Medical Doctor Association
Vice President of the Hematology Branch of the Chinese Society of Geriatrics and Chairman of the Multiple Myeloma Academic Committee
Deputy Leader of the Plasma Cell Group of the Hematology Branch of the Chinese Medical Association
Deputy Leader of the Multiple Myeloma and Related Diseases Professional Group of the Hematology Professional Committee of the Chinese Women Physicians Association
Member of the Chinese and International Primary Systemic Amyloidosis Collaboration Group
Member of the International Myeloma Working Group and the Asia-Pacific Myeloma Working Group

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#Sonrotoclax #RRMM #BCL2Inhibitor #MultipleMyeloma #EHA29 #Hematology #NewTreatment #CancerResearch #2024EHA
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

#EBMT / #EHA Leading the Frontiers of Medicine: China’s Top Medical Expert Explains the Groundbreaking CAR-T Cell Therapy
#EBMT / #EHA Leading the Frontiers of Medicine: China’s Top Medical Expert Explains the Groundbreaking CAR-T Cell Therapy
Expert:**Huang He**
Chief Physician and Doctoral Supervisor.
Currently serves as the Director of the Hematology Institute at Zhejiang University, Director of the Zhejiang Provincial Engineering Research Center for Stem Cell and Cellular Immunotherapy, Chief Scientist for the “Hematology and Immunology Diseases” division at the Liangzhu Laboratory, and Director of the Bone Marrow Transplantation Center at the First Affiliated Hospital of Zhejiang University School of Medicine.
He is the Vice Chairman of the Asia Cell Therapy Organization and Deputy Leader of the Hematopoietic Stem Cell Application Group of the Hematology Branch of the Chinese Medical Association.

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in Chinafor preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in Chinafor preliminary evaluation!
WhatsApp:+8613717959070
Email: doctor.huang@globecancer.com
#MedicalInnovation #CAR_Therapy #Hematology #MedicalBreakthrough #ExpertTalks #CuttingEdgeMedicine #cart #EHA2024
