Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

What Types of Lymphoma Patients Are Suitable for CAR-T Treatment in China?
# What Types of Lymphoma Patients Are Suitable for CAR-T Treatment in China?

Lymphoma
#CART #Lymphoma #DLBCL #LBCL #MedicalTourism #FL #MCL
In recent years, CAR-T cell therapy has sparked a revolution in cancer treatment worldwide, achieving remarkable results, especially in lymphoma care. As one of the global leaders in CAR-T therapy, China has attracted a large number of international patients seeking this cutting-edge immunotherapy. So, what types of lymphoma patients are suitable to travel to China for this advanced treatment?
## Indications: The First Choice for Relapsed or Refractory Lymphoma Patients
CAR-T treatment in China primarily targets patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), and mantle cell lymphoma (MCL). Specific patient groups that are suitable include:
1. **Relapsed DLBCL**: Patients who did not respond to first-line treatments, such as R-CHOP, or relapsed within 12 months after treatment.
2. **Follicular Lymphoma (FL)**: Patients with grade 1-3a FL who have relapsed or are refractory after at least two lines of treatment.
3. **Mantle Cell Lymphoma (MCL)**: Patients whose disease remains difficult to control even after multiple treatments, including BTK inhibitors.
4. Patients whose lymphoma is resistant to traditional treatments or who experience rapid relapse after therapies such as conventional chemotherapy and targeted treatments.
5. More indications include diffuse large B-cell lymphoma (not otherwise specified), DLBCL transformed from follicular lymphoma, primary mediastinal large B-cell lymphoma, and high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangement, as well as grade 3b follicular lymphoma.
CAR-T therapy offers new hope to these patients by targeting the CD19 antigen on the surface of tumor cells.
## Physical Condition: Stable Health is Key
While CAR-T therapy has shown significant efficacy, the potential for serious side effects, such as **cytokine release syndrome (CRS)** and **neurotoxicity**, means that patients must be in stable health to tolerate the risks associated with treatment. Suitable patients should meet the following conditions:
– **Good heart and lung function**: The patient’s cardiovascular and respiratory health should be adequate to handle the stress of treatment.
– **Normal liver and kidney function**: Liver enzymes (such as ALT) and creatinine levels should be within normal ranges to ensure that the patient can properly eliminate toxins from the body.
– **Stable immune system**: Patients should not have severe infections or immunosuppressed conditions, as CAR-T therapy can further weaken the immune system, increasing the risk of infection.
Additionally, patients need to achieve a performance status score of 0-1 on the ECOG scale, indicating that they have good functional ability and can handle daily activities independently.
## Pre-Treatment Testing: Ensuring Proper Antigen Expression
CAR-T therapy targets specific antigens (such as CD19) on the surface of tumor cells, so patients’ cancer cells must express these antigens for treatment to be effective. If tests show that the tumor cells do not express the relevant antigens, CAR-T therapy will not work effectively.
## Costs and Resources: The Advantages of CAR-T in China
Compared to Western countries, CAR-T therapy in China is relatively affordable, usually costing between hundreds of thousands to one million RMB, whereas in the U.S., CAR-T treatment can cost several hundred thousand dollars. For patients with the financial ability but who find it difficult to bear the high costs of treatment in the West, China offers a significant cost advantage.
Moreover, China boasts several top-tier hospitals with established CAR-T treatment programs, such as leading cancer centers in Beijing, Shanghai, and Guangzhou. These hospitals have extensive clinical experience, offering international patients convenient access to treatment.
## Contact Us:
The Advanced Medicine in China team has a wealth of connections with doctors and hospitals, enabling us to quickly help you find the most suitable hospital and expert team for your specific needs. In China, we provide a fast-track option for CAR-T treatment.
## Conclusion
With its cost advantages, advanced medical resources, and broad indications, CAR-T therapy in China has attracted a large number of international lymphoma patients. For those who have failed traditional treatments, are in good physical condition, and have sufficient financial means, CAR-T treatment in China is an ideal choice. If you or someone close to you meets these conditions, consider coming to China for world-leading CAR-T therapy, and open the door to new hope in the fight against cancer.

 To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CancerTherapy #CARTinChina #RelapsedLymphoma #RefractoryLymphoma #FollicularLymphoma #MantleCellLymphoma #Immunotherapy #CancerRevolution #AdvancedCancerCare #CD19Targeting #CancerHope #CancerCare #ChineseHospitals #GlobalHealth #CancerSurvivors #CARTSuccess #MedicalInnovation
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
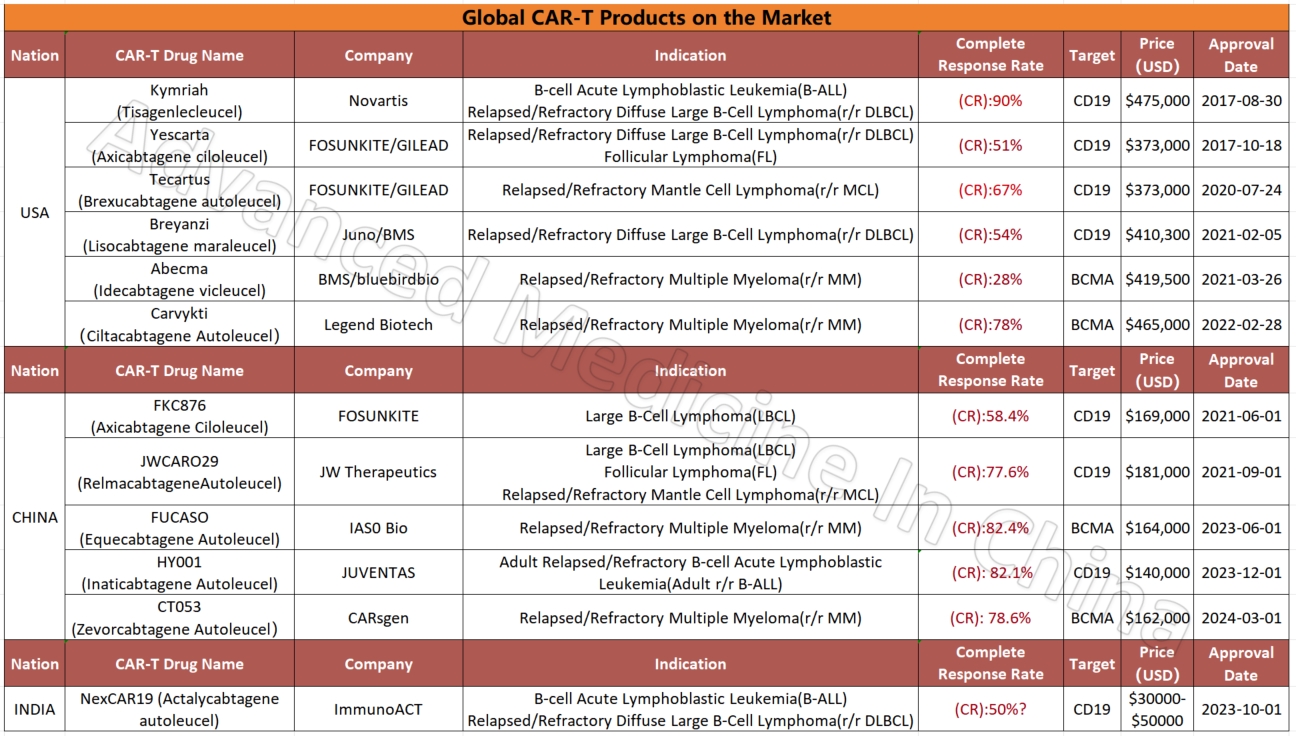
Global CAR-T Products on the Market and BCMA-Targeted CAR-T Products
Global CAR-T Products on the Market

CAR-T
Globally Approved BCMA-Targeted CAR-T Products
– Multiple Myeloma
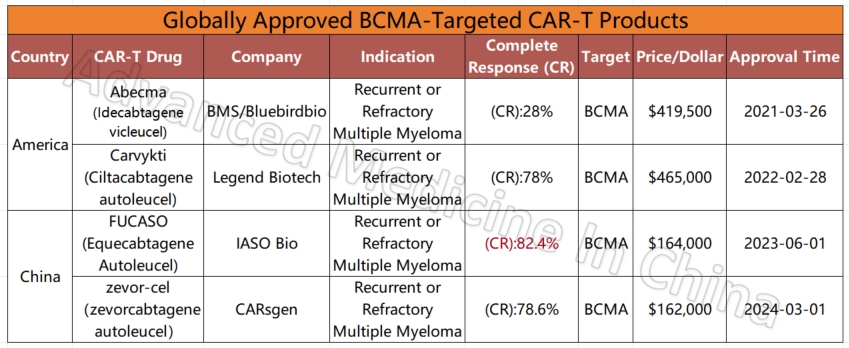
Multiple Myeloma

 To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of Advanced Medicine in China for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China Leads the CAR-T Therapy Revolution, Reshaping the Treatment Landscape of Large B-Cell Lymphoma—2024 CSCO Annual Meeting
**China Leads the CAR-T Therapy Revolution, Reshaping the Treatment Landscape of Large B-Cell Lymphoma—2024 CSCO Annual Meeting**

CSCO
#CSCO #CSCO2024 #Lymphoma #CART #LBCL #RRLBCL #ChinaCAR-T
At the 27th Annual Chinese Society of Clinical Oncology (CSCO) Conference, held in Xiamen, China, CAR-T therapy took center stage as a key focus for the treatment of large B-cell lymphoma (LBCL). As a Chimeric Antigen Receptor T-cell (CAR-T) therapy, this innovative approach is revolutionizing the treatment of relapsed/refractory large B-cell lymphoma (R/R LBCL), offering new hope for patients where traditional therapies have been less effective.
### Breakthroughs in CAR-T Therapy for LBCL
As a major innovation in immunotherapy, CAR-T therapy has shown remarkable efficacy in treating R/R LBCL. Clinical data indicates that about one-third of R/R LBCL patients can achieve long-term remission or even cure through CAR-T therapy. Notably, Axicabtagene ciloleucel (Axi-cel), developed by Fosun Kite Biotechnology, is the first domestically approved CD19-targeted autologous CAR-T product in China. Six-year follow-up results revealed that the two-year overall survival rate was 50.5%, and the six-year lymphoma event-free survival rate was 33.5%.
Meanwhile, Relma-cel, a CAR-T product independently developed by JW Therapeutics in China, has also achieved impressive results. Two-year follow-up data showed a median duration of response of 20.3 months, further highlighting China’s growing research and manufacturing strength in the CAR-T therapy field.
### CAR-T Therapy Expands from Third-Line to Second-Line Use
While CAR-T therapy was initially used only for patients who had failed multiple lines of treatment, it is now being increasingly applied in second-line settings. Recent studies have shown that CAR-T therapy outperforms traditional high-dose chemotherapy and autologous stem cell transplant (ASCT) in certain patient groups. For example, the ZUMA-7 study demonstrated that Axi-cel significantly improved event-free survival (EFS) for LBCL patients treated in the second-line setting, with a median EFS of 8.3 months, compared to 2 months in the ASCT group.
As more research progresses, CAR-T therapy has been recommended by leading clinical guidelines, such as the National Comprehensive Cancer Network (NCCN), for use in patients with primary refractory or early relapsing LBCL. This shift underscores CAR-T’s growing role in earlier stages of treatment, providing more therapeutic options for patients.
### Challenges and Future Prospects
In the exploration of CAR-T as a first-line treatment, promising results are beginning to emerge. The ZUMA-12 study evaluated the use of Axi-cel in high-risk LBCL patients in the first-line setting, with a three-year follow-up showing an overall survival rate of 90.6%. These findings suggest that CAR-T therapy could become an important treatment option for high-risk patients even in the first-line setting.
### China’s Leading Role in CAR-T Therapy
China is rapidly emerging as a global leader in the development and commercialization of CAR-T therapy. With domestically developed products like Axi-cel and Relma-cel achieving significant progress both in China and globally, China’s competitive edge in this field is becoming increasingly evident. As the technology matures and more clinical data are released, the future of CAR-T therapy in LBCL treatment looks exceptionally bright.
The 2024 CSCO Annual Meeting showcased China’s strong commitment to advancing CAR-T therapy. As breakthroughs and challenges coexist, CAR-T therapy is poised to continue playing a critical role in the treatment of large B-cell lymphoma, with China leading the way in this global medical revolution.
To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#LymphomaTreatment #ImmunotherapyRevolution #CancerResearch #MedicalInnovation #BCellLymphoma
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
Chinese Research: Hopes of CAR-T in Second-Line Treatment of LBCL(Lymphoma)
Diffuse Large B-cell Lymphoma (LBCL)is a heterogeneous malignant tumor of the blood system, with approximately 40% of LBCL patients experiencing relapse or developing resistance after first-line treatment, progressing to relapsed/refractory large B-cell lymphoma (R/R LBCL). In recent years, chimeric antigen receptor T-cell therapy targeting CD19 (CAR-T) has made significant breakthroughs in treating relapsed or refractory B-cell non-Hodgkin lymphoma.

At the 65th American Society of Hematology (ASH) Annual Meeting held from December 9th to 12th this year in the Eastern United States, important real-world evidence of CAR-T cell therapy as a second-line standard treatment for R/R LBCL was presented in the form of a poster (ASH Abstract #4876), providing crucial evidence-based medicine for the clinical application of CAR-T.
CAR-T Cell Therapy as Second-Line Treatment for R/R LBCL: Real-World
Evidence Among 110 patients receiving CAR-T infusion, the overall response rate (ORR) was 82.7%, and the complete response (CR) rate was 61.8%. Median progression-free survival (PFS) and overall survival (OS) were not reached. Estimated 6-month PFS and OS rates were 64.1% (95% CI, 52.8%-73.4%) and 84.4% (95% CI, 73.8%-90.9%), respectively.

The advent of CAR-T cell therapy has brought hope of cure for R/R LBCL patients. Based on its critical research and outstanding real-world application data, CAR-T cell therapy has gained recommendations from authoritative guidelines at home and abroad, and has become a crucial choice for second-line treatment in R/R LBCL patients.
#CARTCellTherapy #CancerTreatment #ScienceInnovation #GeneticMedicine #TumorTreatment #HealthcareTech #MedicalScience #CancerAwareness #PatientCare #FutureOfMedicine #CART #LBCL #Lymphoma #bloodcancer
