Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

New Breakthrough in CAR-T Cell Research in China: Type 2 T Cell Function Supports Long-term Leukemia Remission
#### New Breakthrough in CAR-T Cell Research in China: Type 2 T Cell Function Supports Long-term Leukemia Remission

Leukemia
#CAR-T #Leukemia #Type2TCells #ALL #GATA3
In recent years, China has achieved significant progress in CAR-T cell therapy, particularly in treating acute lymphoblastic leukemia (ALL). CD19-targeted CAR-T cell therapy has provided new hope for curing patients with relapsed or refractory ALL. However, some patients experience relapse within a year after treatment, highlighting the need for deeper research into the molecular mechanisms affecting long-term efficacy.
**Key Role of Chinese Medical Team’s Research in Long-term Leukemia Remission**
Recently, a Chinese medical team published a study in *Nature* titled “Single-cell CAR T atlas reveals type 2 function in 8-year leukemia remission,” revealing the crucial role of a specific T cell subtype in the long-term success of CAR-T therapy in leukemia. This study aimed to understand the molecular mechanisms behind the sustained responses observed in some patients receiving CAR-T cell therapy.
The study conducted a comprehensive analysis of CAR-T cells from 82 children with relapsed or refractory ALL and six healthy donors, uncovering the critical role of Type 2 T cell functions in the long-term persistence of CAR-T cells. The findings show that CAR-T cells with Type 2 characteristics demonstrate remission lasting over eight years after treatment.
**T Cell Cytokines Enhance the Long-term Activity of CAR-T Cells**
The research team found that an increase in Type 2 T cell cytokines (such as IL-4, IL-5, and IL-13) helps maintain CAR-T cells’ antitumor activity. This effect is driven by the key regulatory factor GATA3, whose upregulation significantly enhances CAR-T cell memory and persistence, reduces cell dysfunction, and improves treatment durability.
In animal model experiments, CAR-T cells with higher Type 2 characteristics demonstrated stronger tumor-clearing abilities and memory properties. Incorporating Type 2 cytokines into CAR-T cell manufacturing not only boosted the cells’ anticancer functions but also enhanced their efficacy and persistence over long-term treatments. Proteomic analysis of patients in sustained remission revealed increased levels of Type 2 cytokines, particularly interleukin-13, suggesting that an immune environment rich in Type 2 cytokines contributes to CAR-T cell persistence. In leukemia mouse models, Type 2 high-expression CAR-T cells displayed enhanced tumor-clearing ability, expansion capacity, and memory responses.
**In Conclusion, Valuable Insights into the Mechanism of Long-term CAR-T Cell Therapy for Leukemia**
This study offers valuable insights into the mechanisms behind the long-term persistence of CAR-T cell therapy for leukemia. The results suggest that enhancing Type 2 T cell function, particularly through GATA3 regulation, may significantly improve the persistence and efficacy of CAR-T cell therapy.
**China’s CAR-T Cell Research Supports Global Cancer Combat**
This study provides a new perspective for CAR-T cell therapy, with Chinese scientists’ technological breakthroughs showing great potential in enhancing the durability of leukemia treatments. This advancement will offer stronger treatment options for cancer patients globally and lay a solid foundation for the future of cancer immunotherapy.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#LeukemiaResearch #CancerImmunotherapy #ALLTreatment #CancerBreakthrough #ChinaMedicalResearch #CellTherapy #CancerRemission #PrecisionMedicine #Oncology
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Groundbreaking Chinese CAR-T Research: CD5 CAR-T Therapy Brings New Hope for Relapsed/Refractory T-cell Acute Lymphoblastic Leukemia (T-ALL)
Groundbreaking Chinese CAR-T Research: CD5 CAR-T Therapy Brings New Hope for Relapsed/Refractory T-cell Acute Lymphoblastic Leukemia (T-ALL)

T-ALL
Relapsed or refractory acute T-cell lymphoblastic leukemia (r/r T-ALL) has long lacked effective treatment options, with a poor prognosis. Although CD7 CAR-T therapy has shown some efficacy, many patients experience relapse due to CD7 antigen loss.
#CAR-T #TALL #ALL #CARTtherapy #leukemia #NatureMedicine #Tcell #CD5
Breakthrough Research: CD5 CAR-T Therapy Published in Nature Medicine
To address this challenge, a team of Chinese medical professors published groundbreaking research in Nature Medicine titled “Allogeneic CD5-specific CAR-T therapy for relapsed/refractory T-ALL: a phase 1 trial.” This study explores the application of donor-derived CD5 CAR-T therapy in r/r T-ALL, offering a new possibility for the treatment of T-cell hematologic malignancies.
Study Design: Exploring the Safety and Efficacy of CAR-T Therapy
This study focused on a 21-day dose-limiting toxicity (DLT) observation period and adverse reactions within 30 days, preliminarily confirming the short-term safety of CD5 CAR-T therapy. Excitingly, early results showed complete remission in all enrolled patients, demonstrating significant short-term efficacy. Despite this positive progress, the research team also observed that, in long-term follow-up, patients who did not receive bridging transplantation faced a risk of functional deficits due to T-cell insufficiency. Addressing this challenge will be a key focus for the next stage of optimization.
Future Outlook: Exceptional Progress by the Chinese Team Brings New Hope to Patients
In future research, the Chinese medical and scientific team will continue efforts to enhance the safety of CAR-T therapy, particularly by adding “switch” components to allow flexible activation or deactivation of the therapy in patients, aiming for durable efficacy. If successful, this optimization could greatly expand the potential of CAR-T therapy in treating T-cell malignancies.
This research not only brings hope to patients with relapsed/refractory T-ALL but also signifies outstanding progress by the Chinese medical and research team in innovative therapies. The work of Professor Pan and his team highlights the leading position of China’s CAR-T therapy in global cancer immunotherapy and holds promise for bringing new life-saving options to more patients with T-cell hematologic malignancies.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#TALLTreatment #Immunotherapy #CancerResearch #Breakthrough
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Breakthrough in China’s CAR-T Therapy for Treating Relapsed B-ALL: Donor-Derived CD19 CAR-T Shows Long-Term Survival Advantage
**Breakthrough in China’s CAR-T Therapy for Treating Relapsed B-ALL: Donor-Derived CD19 CAR-T Shows Long-Term Survival Advantage**

B-ALL
#CAR_TTherapy #B_ALL #DonorCAR_T #CAR_T #ALL #ChinaCART #CD19 #alloHSCT
In recent years, China has made significant strides in CAR-T cell therapy, particularly in treating B-cell acute lymphoblastic leukemia (B-ALL). Donor-derived CAR-T therapy has shown promising efficacy for B-ALL patients who relapse after allogeneic hematopoietic stem cell transplantation (allo-HSCT), bringing new hope for long-term survival.
A study jointly published by the Chinese Academy of Medical Sciences and Chinese medical teams in the *Journal of Hematology & Oncology*, titled “Long-term survival with donor CD19 CAR-T cell treatment for relapsed patients after allogeneic hematopoietic stem cell transplantation,” indicates that patients treated with donor CD19 CAR-T cells achieved complete remission (CR) without requiring a second transplant, with a significant increase in long-term survival rates. This study followed 32 B-ALL patients who relapsed post-allo-HSCT. The median patient age was 24, and after receiving donor CD19 CAR-T therapy, they achieved complete remission or partial recovery in peripheral blood (CRi), with a median follow-up of 42 months.
Results showed that patients treated with donor CD19 CAR-T had a 2-year overall survival (OS) rate of 56.25% and an event-free survival (EFS) rate of 50.0%. The 5-year OS and EFS reached 53.13% and 46.88%, respectively, with no new long-term adverse events. These findings suggest that donor CAR-T cells are not only effective but also have long-term safety advantages over second transplantation or traditional donor lymphocyte infusion, offering a more promising treatment option for relapsed B-ALL patients.
While these results are encouraging, further development of early detection methods is needed to identify or prevent relapse at an earlier stage. Early relapse with donor CAR-T, especially within the first six months post-treatment, remains a challenge, requiring additional multi-center, prospective studies for validation. Overall, this research provides robust clinical data supporting donor CAR-T therapy for relapsed B-ALL patients post-transplant, potentially establishing a benchmark for CAR-T therapies in China on a global scale.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#ChinaMedicalBreakthrough #CancerResearch #Hematology #Oncology #LongTermSurvival #StemCellTransplantation #RelapsedLeukemia #ChineseMedicalResearch
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China’s CAR-T Therapy: A Beacon of Hope for Children with Acute Lymphoblastic Leukemia (ALL)
China’s CAR-T Therapy: A Beacon of Hope for Children with Acute Lymphoblastic Leukemia (ALL)

#CARTTherapy #Leukemia #ChildhoodCancer #ALL #CART #AcuteLymphoblasticLeukemia #Patientstory
China’s CAR-T cell therapy is emerging as a beacon of hope in the fight against cancer, bringing unprecedented treatment possibilities. As a precise cellular immunotherapy, CAR-T therapy enhances the cancer-fighting abilities of T-cells through genetic editing technology, offering breakthroughs for patients with cancers resistant to traditional treatments, especially in blood cancers like leukemia.
From Diagnosis to Treatment: Strength and Struggle
Shan is a young child in China who has endured long-term treatment for leukemia. Diagnosed with acute lymphoblastic leukemia (ALL) in 2019, this news brought immense suffering to her and her family, as she was only five years old. Although ALL generally responds well to chemotherapy, Shan’s cancer cells persisted stubbornly after several rounds of chemotherapy, failing to reach full remission. Her doctors recommended a bone marrow transplant, but due to physical and family constraints, she had to opt for conservative treatment. Nevertheless, Shan did not give up her desire to live, showing remarkable resilience over five years.
A New Hope Through CAR-T Therapy
In 2023, Shan’s condition worsened as her anemia grew severe, with her hemoglobin levels dropping to a critical point. Faced with despair, her mother reached out to our team at Advanced Medicine in China, where experts quickly admitted her to the hospital. After a comprehensive evaluation, our hematology-oncology specialists decided to proceed with CAR-T therapy for her.
Following a thorough process of gene modification and the infusion of CAR-T cells, Shan’s condition improved rapidly. In the early stages of treatment, her complexion brightened, her energy levels noticeably improved, and subsequent tests showed that her tumor cells had completely disappeared, achieving clinical remission. Shan was finally free from the shadow of cancer, gradually regaining the joy and health of childhood.
A Medical Breakthrough: New Hope for Children with Leukemia
Shan’s success story signifies the maturation of CAR-T therapy in China, showcasing its powerful potential, particularly in treating refractory and relapsed childhood acute leukemia. In recent years, Chinese medical teams have actively adopted CAR-T technology in treating pediatric blood cancers, achieving remarkable long-term remission for many children. As the country with the highest number of CAR-T therapy applications globally, China’s hospital teams possess extensive experience and advanced technological capabilities, offering high-quality treatment to more children suffering from relapsed and refractory blood diseases.
Moving Forward: A Promising Future for Cellular Immunotherapy
With the continued development of CAR-T therapy in China, more patients with challenging cancers stand to benefit. Shan’s recovery is not only a family’s joy but also a testament to China’s medical progress. CAR-T therapy is ushering in a new era of cellular immunotherapy, giving more patients the possibility of life extension and recovery.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+861371795907
Email: doctor.huang@globecancer.com
#CancerResearch #MedicalBreakthrough #Immunotherapy #ChinaMedicine #Oncology #CancerHope #LeukemiaAwareness #CancerTreatment
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China’s New CAR-T Therapy: Bringing New Hope for Acute Myeloid Leukemia (AML)
**China’s New CAR-T Therapy: Bringing New Hope for Acute Myeloid Leukemia (AML)**

AML
#CLL1CART #AML #Leukemia #CART #CARTtherapy #AcuteMyeloidLeukemia #CLL1 #CD371
In recent years, China’s rise in the field of medical innovation has been remarkable, especially with breakthroughs in cancer and hematologic malignancy treatments that are drawing global attention. Today, we are introducing a cutting-edge treatment from China—donor-derived CLL-1 CAR-T therapy, which offers new hope for adults with relapsed or refractory acute myeloid leukemia (AML). This advanced therapy provides a novel option when conventional treatments prove ineffective.
In AML treatment, the CLL-1 antigen (also known as CD371) is an ideal target, as it is highly expressed on AML leukemic stem cells but not on healthy hematopoietic stem cells. CAR-T therapy targeting CLL-1 can precisely attack AML cells while minimizing effects on healthy immune cells, offering a specific treatment choice with fewer side effects.
### Success Cases of Donor-Derived CLL-1 CAR-T Therapy in China
A 17-year-old Chinese high school student successfully underwent donor-derived CLL-1 CAR-T treatment, providing critical evidence of the therapy’s potential. After being diagnosed with AML, he went through multiple rounds of chemotherapy and autologous stem cell transplantation, but frequent relapses made treatment exceedingly difficult. Due to a lack of sufficient lymphocytes for autologous CAR-T therapy, doctors decided to use donor-derived CLL-1 CAR-T therapy, followed by an allogeneic stem cell transplant.
– **Treatment Process:** After successfully collecting donor lymphocytes and completing preconditioning, the patient received two CAR-T cell infusions. Initially, he experienced mild fever, a common reaction to cytokine release syndrome (CRS), but his condition soon improved significantly.
– **Remarkable Effectiveness:** On the eighth day post-infusion, bone marrow testing showed no cancer cells, lab results returned to normal, and he successfully completed the allogeneic stem cell transplant. During follow-up, his condition remained stable without signs of relapse, and he has since returned to school, resuming a normal life.
### The Breakthrough Significance of China’s CAR-T Therapy in Global AML Treatment
Chinese experts believe that China’s donor-derived CLL-1 CAR-T therapy has significant value in AML treatment, particularly for patients who are unable to undergo conventional CAR-T therapy due to insufficient lymphocytes. Allogeneic stem cell transplantation is an effective AML treatment, but the high relapse rate remains challenging. Introducing CAR-T therapy before transplantation can significantly reduce the risk of relapse, extend patient survival, and improve quality of life. Most patients tolerate the therapy well, with only mild adverse reactions, such as CRS and minor neurotoxicity, and long-term follow-up shows durable effectiveness.
China’s breakthrough in CAR-T therapy for AML provides a new model for leukemia treatment worldwide. China’s donor-derived CLL-1 CAR-T therapy not only brings hope to patients but also offers valuable insights to the global medical community in leukemia treatment. As medical technology advances, Chinese CAR-T therapy will bring benefits to more leukemia patients, helping them achieve long-term survival and improved quality of life.
To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of Advanced Medicine in China for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#MedicalInnovation #CancerTreatment #HematologicMalignancy #LeukemiaTreatment #ChinaBiotech #CellTherapy #StemCellTransplant #GlobalHealth
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Significant Progress in CAR-T Therapy in China: Long-Term Effects of Combined CD19 and CD22 Treatment for Acute B-Lymphoblastic Leukemia
### Significant Progress in CAR-T Therapy in China: Long-Term Effects of Combined CD19 and CD22 Treatment for Acute B-Lymphoblastic Leukemia

Leukemia
#ALL #CAR-Ttherapy #Leukemia #CancerResearch #B_ALL #LeukemiaTreatment
Recently, a Chinese medical team published a notable study titled “Five-year outcome of CD19 combined with CD22 CAR-T cell therapy in B-ALL patients relapsed after allo-transplantation.” The research highlights the long-term efficacy of combined CD19 and CD22 CAR-T cell therapy in patients with relapsed acute B-lymphoblastic leukemia (B-ALL) after allogeneic hematopoietic stem cell transplantation (allo-HCT). This breakthrough has not only brought new hope to B-ALL patients but also attracted significant global attention.
**Background and Significance**
Acute B-lymphoblastic leukemia is a hematologic malignancy with poor prognosis, especially for patients who experience relapse after allo-HCT, where survival rates are significantly reduced. CAR-T cell therapy in China has shown increasingly positive results in treating B-ALL, particularly in targeting the CD19 antigen. However, the effects of targeting the CD22 antigen and its potential in combination with CD19 therapy are still under deeper investigation.
**Study Design and Methodology**
Based on a previous phase I clinical trial, this study involved a follow-up of 27 patients who had received CD19 CAR-T treatment. To comprehensively assess the treatment’s efficacy, the study also included three additional patients who experienced relapse with minimal residual disease (MRD) in the bone marrow. Although these patients did not meet the initial trial’s criteria, they received combined CD19 and CD22 CAR-T cell therapy under the same protocol. The CAR-T cells used in this study were second-generation designs created via lentiviral vector transfection.
**Study Results**
After a 5-year follow-up, the Chinese research team found that combined CD19 and CD22 therapy significantly improved patients’ long-term survival rates. Among the 30 patients who completed the combined therapy, the median follow-up time was 64.4 months. After two complete treatment cycles, patients maintained sustained remission. Survival analysis showed that the 3-year and 5-year overall survival rates reached 79% and 75%, respectively, with event-free survival rates of 54% and 50%. These results indicate that combined CD19 and CD22 CAR-T cell therapy offers substantial long-term efficacy for relapsed B-ALL patients.
**Conclusions and Future Outlook**
This study not only validates the potential of CAR-T cell therapy in hematologic malignancies but also provides new therapeutic insights for clinical practice. The combination of CD19 and CD22 holds promise for offering a more effective treatment option for B-ALL patients who relapse after allo-HCT, significantly improving their long-term survival rates.
As research in CAR-T cell therapy deepens, we may see more targeted approaches and optimized treatment protocols emerge. China’s active exploration and innovation in this field bring renewed hope to hematologic cancer patients worldwide, with the expectation that CAR-T therapy will further improve survival rates and quality of life for B-ALL patients in the near future.
To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of Advanced Medicine in China for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#Immunotherapy #CD19CD22Combo #StemCellTransplant #ChinaMedicalResearch #Hematology #CancerBreakthrough #LongTermSurvival #BloodCancer
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Another New CAR-T Therapy in China is Fast-Tracking Approval, Targeting Pediatric Acute Lymphoblastic Leukemia(ALL) with a 100% Response Rate!
Another New CAR-T Therapy in China is Fast-Tracking Approval, Targeting Pediatric Acute Lymphoblastic Leukemia with a 100% Response Rate!

ALL
In recent years, CAR-T cell therapies from China have garnered global attention in the fight against cancer, and now another CAR-T product is about to hit the market. Priscabtagene Autoleucel Injection (pCAR-19B), developed by China’s Precision Biotech, is specifically designed for relapsed or refractory B-cell acute lymphoblastic leukemia (ALL) and is currently under priority review for approval. It is poised to become China’s first CAR-T therapy specifically tailored for children and adolescents with acute leukemia.
This groundbreaking therapy has shown remarkable efficacy, achieving a 100% overall response rate in early clinical trials. For patients who no longer respond to conventional treatments, Priscabtagene Autoleucel offers a new lifeline. The therapy has demonstrated superior safety and efficacy in clinical settings, thanks to gene optimization and advanced vector systems that reduce side effects while enhancing effectiveness.
Currently, pCAR-19B has entered Phase II clinical trials, expanding its scope beyond children to include adult patients with relapsed leukemia. It is also being researched for the treatment of other malignant lymphomas, such as diffuse large B-cell lymphoma and follicular lymphoma. This signifies the vast potential of CAR-T therapies in China, promising more effective treatment options for blood cancer patients both domestically and globally.
Precision Biotech’s CAR-T therapy is expected to gain approval soon, contributing to global medical innovation while bringing new hope to countless patients.
To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of Advanced Medicine in China for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

How to Choose Among Similar CAR-T Therapies?
**How to Choose Among Similar CAR-T Therapies?**
Currently, there are multiple CAR-T products targeting the same antigen available on the market, which can make it difficult for patients to decide which one to choose.
Experts recommend selecting a therapy based on the treatment target. It is generally advisable to prioritize approved products, as they have been validated, providing better assurance of safety and efficacy. For example, CD19-targeting CAR-T products are typically preferred over CD22-targeting ones, and BCMA-targeting therapies are favored over GPRC5D-targeting ones.
Additionally, it’s important to consider the origin of the CAR-T cells (such as human-derived vs. mouse-derived) and the costimulatory signals used (e.g., 4-1BB vs. CD28). Different conditions may also influence the choice; for instance, lymphoma patients may be more suitable for CD28, while those with acute leukemia might benefit more from 4-1BB.
In the end, patients are advised to make an informed decision based on their medical history and the guidance of their doctors.

 To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of Advanced Medicine in China for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
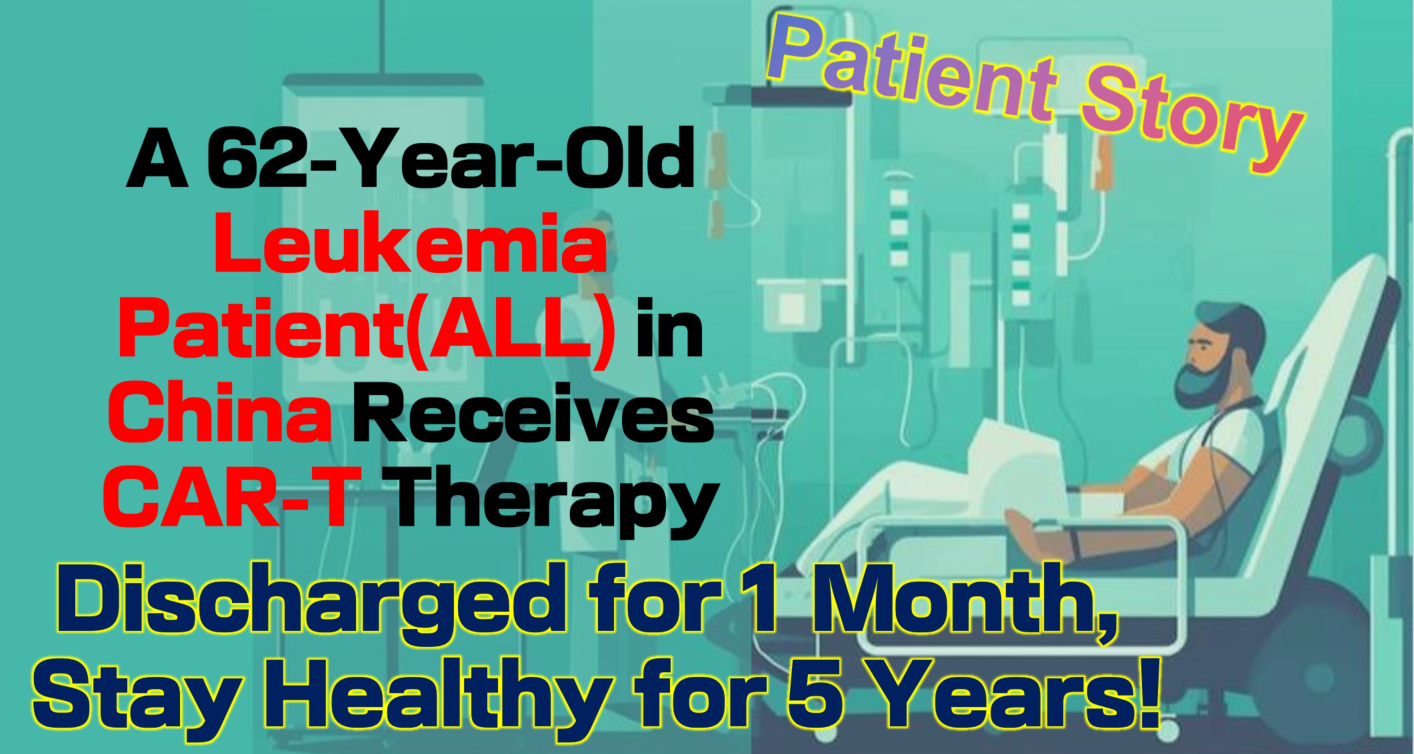
A 62-Year-Old Leukemia Patient in China Receives CAR-T Therapy, Discharged After One Month, and Still Healthy After Five Years!
A 62-Year-Old Leukemia Patient in China Receives CAR-T Therapy, Discharged After One Month, and Still Healthy After Five Years!

Patient Story
#Leukemia #CAR_Therapy #CancerSurvivor #ChineseHealthcare#PatientStory
Acute lymphoblastic leukemia (ALL) is a type of leukemia that originates from B or T lymphoid progenitor cells. Leukemia cells proliferate abnormally in the bone marrow, suppressing normal hematopoiesis, leading to anemia, thrombocytopenia, and neutropenia.
Patient Story:
In 2019, 62-year-old Mr. Ke from China sought treatment at multiple hospitals due to weakness and fatigue, but no diagnosis was made. After being transferred to the hematology department and undergoing a bone marrow biopsy, he was finally diagnosed with acute lymphoblastic leukemia (ALL). Despite undergoing numerous chemotherapy sessions, his condition worsened, and his health deteriorated further.
In this challenging situation, with the help of the Advanced Medicine In China team, Mr. Ke transferred to a renowned hospital in Guangdong, China, to try the advanced CAR-T cell therapy. This was a bold decision after two years of failed chemotherapy attempts.
CAR-T Therapy: A New Hope for Leukemia Treatment
CAR-T cell therapy is a groundbreaking treatment that modifies a patient’s T cells to recognize and attack cancer cells. In early 2019, Mr. Ke received CD19 CAR-T therapy combined with a tumor DC vaccine. His T cells were reprogrammed and reinfused to fight leukemia cells.
The treatment process wasn’t easy. Although initially planned for discharge within days, he experienced high fever and lung infections during hospitalization. However, with timely intervention from the Chinese medical team, these complications were effectively managed. Eventually, Mr. Ke was discharged, and follow-up tests confirmed his complete remission.
Surpassing Traditional Therapies and Embracing a New Life
For ALL, chemotherapy is more effective in children, but its efficacy is often limited in adults, especially high-risk patients. Mr. Ke recalls, “After countless failed chemotherapy sessions, I was almost hopeless. Fortunately, CAR-T therapy worked like a miracle.”
Today, five years later, Mr. Ke remains in good health, fully recovered. Chinese hematology experts noted, “For patients who receive early CAR-T treatment, the five-year survival rate now exceeds 70%. We hope that more patients can benefit from this advanced therapy, regain their health, and enjoy life again.”
China’s advancements in CAR-T cell therapy have garnered global attention, with many international patients seeking this cutting-edge treatment. Mr. Ke’s recovery story brings hope to countless patients and highlights China’s innovative achievements in immunotherapy.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CancerTreatment #Immunotherapy #MedicalBreakthrough #AdvancedMedicine #HealthRecovery
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

At which stage of treatment is the best time to choose CAR-T therapy?
At which stage of treatment is the best time to choose CAR-T therapy?
CAR-T therapy is often used as a last resort, but it should be introduced earlier for better outcomes. Early use, even as a frontline or 1.5-line treatment, could dramatically increase patient cure rates.
Tumor cells are highly adaptable and intelligent, learning to evade treatments over time. Treating tumors early, before they become stronger, is crucial.
CAR-T should not be considered a “last resort” in treatment, as waiting too long may reduce its effectiveness. It’s better to “kill the tumor when it’s young” before it becomes more resilient and difficult to eliminate.

 To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of Advanced Medicine in China for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Why Are More and More International Patients Coming to China for CAR-T Cell Therapy?
Why Are More and More International Patients Coming to China for CAR-T Cell Therapy?

CARTtherapy
#CARTtherapy #InternationalPatient #CART #Multiplemyeloma #lymphoma #leukemia
In recent years, CAR-T cell therapy, as an innovative cancer treatment, has rapidly gained widespread attention globally. China, as a leading country in the CAR-T treatment field, has attracted an increasing number of patients from Europe and Asia due to its advanced technology, high-quality medical resources, and relatively lower treatment costs. So, why are more international patients choosing to come to China for CAR-T treatment? Here are some key reasons worth noting:
### 1. **Advanced CAR-T Treatment Technology**
Over the past decade, China has made tremendous strides in medical research, particularly in the field of cancer immunotherapy. Not only has China successfully developed various CAR-T therapies, but it has also built a comprehensive treatment protocol and safety monitoring system to ensure efficient and safe treatment for patients. Additionally, China has accumulated extensive experience in managing treatment-related side effects, such as CRS (Cytokine Release Syndrome) and neurotoxicity.
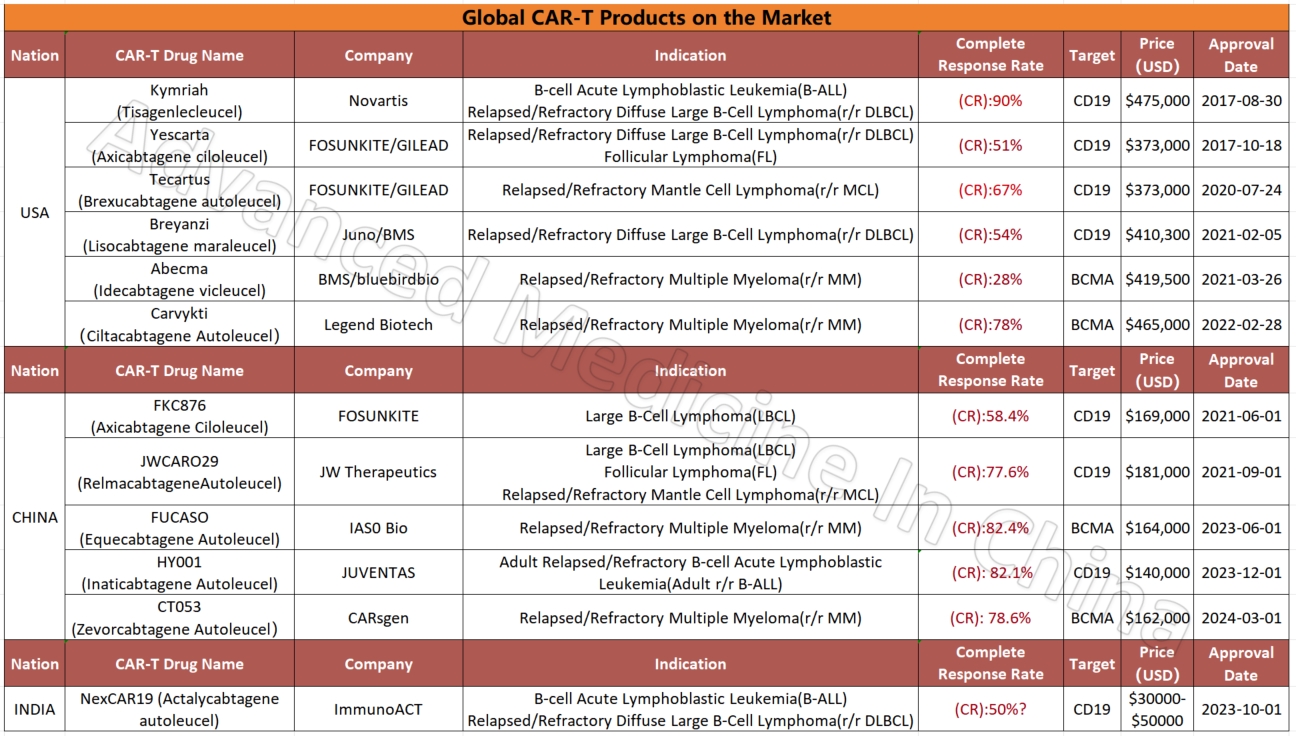
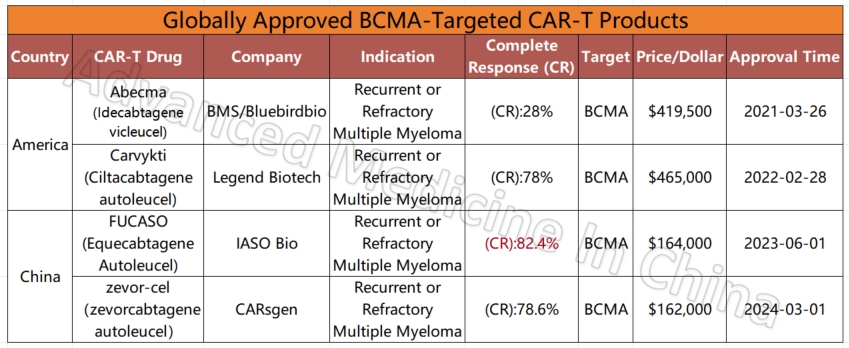
Currently, more than 50% of the world’s clinical trials for CAR-T therapy are conducted in China. Of the 11 CAR-T products already on the global market, five are from China. These include products for multiple myeloma (BCMA CAR-T), diffuse large B-cell lymphoma (CD19 CAR-T), and leukemia. Compared to similar products in the U.S. for multiple myeloma, China’s CAR-T products are not only fully humanized but also boast the highest complete remission rates among all available products, reaching 82.4%. The CR data for China’s lymphoma and leukemia CAR-T products are also impressive, at 77.6% and 82.1%, respectively.
### 2. **International Certification and Clinical Trial Opportunities**
Several hospitals and research institutions in China have gained international recognition for their CAR-T therapies. Patients can choose between commercial CAR-T treatments or participate in clinical trials. For some CAR-T treatments that have not yet been approved or are hard to access in Europe or Asia, patients can receive these cutting-edge therapies in China earlier.
Just a few days ago, Chinese scholars’ research on universal CAR-T cell therapy was featured on the cover of *Nature*. Various other studies on Chinese CAR-T therapies are frequently published in major medical journals. For example, a Chinese medical team published a study on dual-target CAR-T in *JAMA Oncology*, showing a 100% stringent complete remission (sCR) rate in high-risk multiple myeloma patients. Additionally, a research team from Zhejiang, China, published clinical data in *Cell Discovery*, highlighting the breakthrough of the fourth-generation anti-CD19 CAR-T cell therapy in treating relapsed/refractory large B-cell lymphoma with exceptional anti-tumor capabilities.
### 3. **Relatively Low Treatment Costs**
Compared to the million-dollar treatment costs in Western countries, CAR-T treatment in China is relatively affordable, often costing just 1/5 to 1/7 of the price in the U.S. For instance, the cheapest CAR-T product for multiple myeloma in the U.S. costs $420,000, and when including hospitalization and other expenses, the total cost can reach around $700,000. In contrast, similar Chinese products, with equal or even better effectiveness, cost only $160,000, and hospitalization and other fees are much lower, often under $10,000. Some treatment options are available for just a few tens of thousands of dollars, and in certain cases, treatment is even completely free.
Patients from Europe and Asia can receive world-class CAR-T therapy in China at a much lower cost, providing an affordable option for those who find the high costs of treatment elsewhere prohibitive. Moreover, a range of one-stop medical services, including hospital visits, translation assistance, transportation, accommodation, and even family care, can significantly enhance the patient experience for international patients.
### 4. **World-Class Expert Teams**
China is home to a group of internationally renowned oncology and immunotherapy experts who have extensive clinical experience and academic achievements in hematological cancers and CAR-T therapy. Patients receive personalized treatment plans designed by leading experts, ensuring that each treatment is precisely tailored to their specific condition, maximizing effectiveness and minimizing risks.
For example, Professor Huang Xiaojun, Director of the Institute of Hematology at Peking University, is one of the world’s pioneers in haploidentical transplantation, having developed China’s haploidentical hematopoietic stem cell transplantation model. His innovative approach has increased the 3-year survival rate of leukemia patients receiving haploidentical transplants from around 20% to approximately 70%, earning him the Distinguished Service Award from the Center for International Blood and Marrow Transplant Research.
### 5. **Efficient and Convenient Medical Services**
China has implemented a 144-hour visa-free transit policy for citizens of 54 countries and mutual visa exemptions with 24 countries. This is a major benefit for international patients seeking treatment in China. Moreover, China’s healthcare system is known for its efficiency and convenience, especially in treating major diseases. Appointment wait times are short, and there is minimal delay in receiving treatment. A “super green channel” is also available for international patients, ensuring quick diagnosis and treatment planning. From initial consultation to online meetings with specialists, the process typically takes less than a week, with hospital admission and treatment beginning within another week.
Compared to the long waiting periods in other countries, China’s efficient medical services significantly improve treatment success rates and patient satisfaction. CAR-T therapy, in particular, is known for its high efficiency. There was an international patient who went from initial consultation to complete remission and a safe return home in less than a month—a remarkable achievement considering CAR-T is a “living” drug that must be custom-prepared using the patient’s own blood cells.
### 6. **Abundant Success Stories**
Many international patients who have received CAR-T therapy in China have achieved remarkable results, with their cancers being effectively controlled or even cured. These success stories not only boost confidence among patients worldwide but also attract more people to China for treatment. By sharing these stories, more patients learn about China’s advantages in CAR-T therapy and choose it as their treatment destination.
For instance, Ethan, a patient from Singapore, achieved complete remission just four weeks after CAR-T treatment and has since recovered very well. There is also the story of a Russian patient with high-risk, late-stage multiple myeloma who received efficient treatment in China at a much more reasonable cost and with a faster treatment process.
### 7. **Conclusion**
In conclusion, as China continues to enhance its technological capabilities in CAR-T cell therapy, more and more patients from around the world are choosing to come to China for this cutting-edge cancer treatment. With its advanced medical technology, international services, and relatively lower treatment costs, China is becoming an important destination for cancer patients seeking treatment. If you or a loved one is searching for innovative cancer treatment options, consider CAR-T therapy in China for a chance at recovery!
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can contact us for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CellTherapy #CancerTreatment #Immunotherapy #HealthcareInnovation #MedicalTourism #ChinaHealthcare #CancerSurvivor #PatientStories #MedicalAdvancements #AffordableHealthcare #PersonalizedMedicine #GlobalHealth #HealthJourney #Oncology
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
Global CAR-T Products on the Market and BCMA-Targeted CAR-T Products
Global CAR-T Products on the Market

CAR-T
Globally Approved BCMA-Targeted CAR-T Products
– Multiple Myeloma
Multiple Myeloma
