The new hope for pediatric patients with relapsed/refractory B-cell acute lymphoblastic leukemia (R/R B-ALL) boasts an overall survival rate of up to 96%.
The new hope for pediatric patients with relapsed/refractory B-cell acute lymphoblastic leukemia (R/R B-ALL) boasts an overall survival rate of up to 96%.

Recently, CAR-T cell therapy targeting B-cell malignancies has encountered a series of inquiries and challenges, particularly concerning discussions on CAR-T cell-related toxicity, resistance, antigen escape, and limitations in persistence. However, a groundbreaking concept addressing relapse in patients after CAR-T cell therapy has been introduced for the first time: a sequential approach involving distinct targeted CAR-T cell therapies.
Within this approach, CD19 CAR-T cell therapy has demonstrated the ability to achieve complete remission in 60% to 90% of relapsed or refractory acute B-cell lymphoblastic leukemia patients. By experimenting with different combinations and sequential administration strategies of B-cell antigen-targeted CAR-T cell therapies, there’s potential to prevent tumor antigen escape and prolong the persistence of CAR-T cells.
Preliminary clinical trials have provided initial support for this concept, notably a phase II clinical trial aimed at assessing the efficacy of sequential CD19 and CD22 CAR-T cell therapy. Its findings revealed a 79% event-free survival rate, an 80% sustained remission rate, and an impressive 96% overall survival rate among patients receiving targeted doses in sequential therapy. Encouragingly, the overall safety of this sequential therapy appeared manageable, providing long-term survival benefits for children with relapsed or refractory acute B-cell lymphoblastic leukemia.
However, the limitations of antigen escape and limited persistence after CAR-T cell therapy persist. Addressing these challenges, researchers have proposed the hypothesis of sequential administration of CAR-T cell products targeting different antigens, aiming to maintain the persistence of CAR-T cells.
The results of this phase II clinical trial indicate that administering CD22 CAR-T cell therapy following CD19 CAR-T cell infusion can result in longer-lasting remission effects for pediatric patients with relapsed or refractory B-cell acute lymphoblastic leukemia, achieving an 80% sustained remission rate over 18 months and an impressive 96% overall survival rate. Importantly, the overall safety of this sequential therapy is uplifting, providing long-term survival benefits for this specific patient population.
In summary, this study presents groundbreaking evidence for new strategies and directions in CAR-T cell therapy. Despite existing limitations, this therapy demonstrates significant potential in treating uncontrollable acute B-cell lymphoblastic leukemia, potentially offering more enduring treatment effects and long-term survival benefits for these patients. This achievement points towards a viable path for the future development of cell therapies.
This Phase 2 trial, conducted at Beijing GoBroad Boren Hospital in China, enrolled pediatric patients aged 1–18 years diagnosed with relapsed or refractory B-cell acute lymphocytic leukaemia (ALL) showing CD19 and CD22 positivity exceeding 95%.

If a child unfortunately gets leukemia, should CAR-T Therapy be considered?
In recent years, CAR-T therapy has gained traction in treating leukemia. A quick search yields numerous articles, many of which describe the miraculous effects of CAR-T.
Is the efficacy of CAR-T therapy really that impressive? Should CAR-T therapy be considered for a child diagnosed with leukemia? Today, let’s unravel these doubts together!
What kind of leukemia patients can undergo CAR-T therapy?
Since most acute leukemia patients are sensitive to chemotherapy, chemotherapy is the preferred initial treatment. Currently, CAR-T therapy is primarily used for refractory and relapsed acute B-cell lymphoblastic leukemia patients.
For cases where 1-2 courses of chemotherapy fail to achieve complete remission (primary refractory), or relapse during chemotherapy, or experience ineffective re-treatment after relapse and re-chemotherapy (refractory relapse), CAR-T therapy is the preferred approach.
Celebrating a decade of being cancer-free for the world’s first leukemia child treated with CAR-T therapy
The world’s first leukemia child cured by CAR-T therapy celebrates 11 cancer-free years
On May 10th each year, Emily Whitehead commemorates the anniversary of her cancer-free survival. This year, she turns 18 and has officially become a nurse, leading a busy and joyful life. With the achievement of this significant milestone, the revolutionary cancer treatment known as CAR-T cell immunotherapy has been officially recognized! She has also become the spokesperson for this epic therapy.
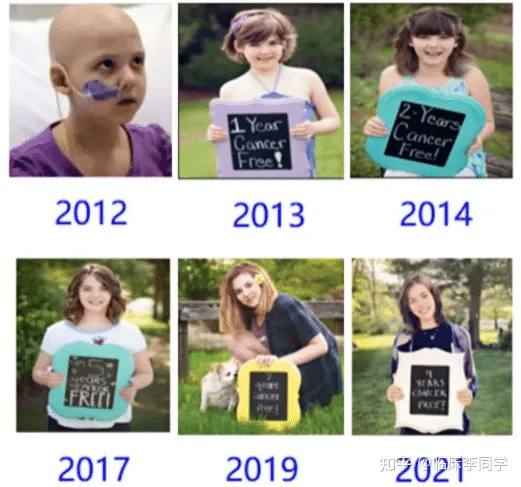
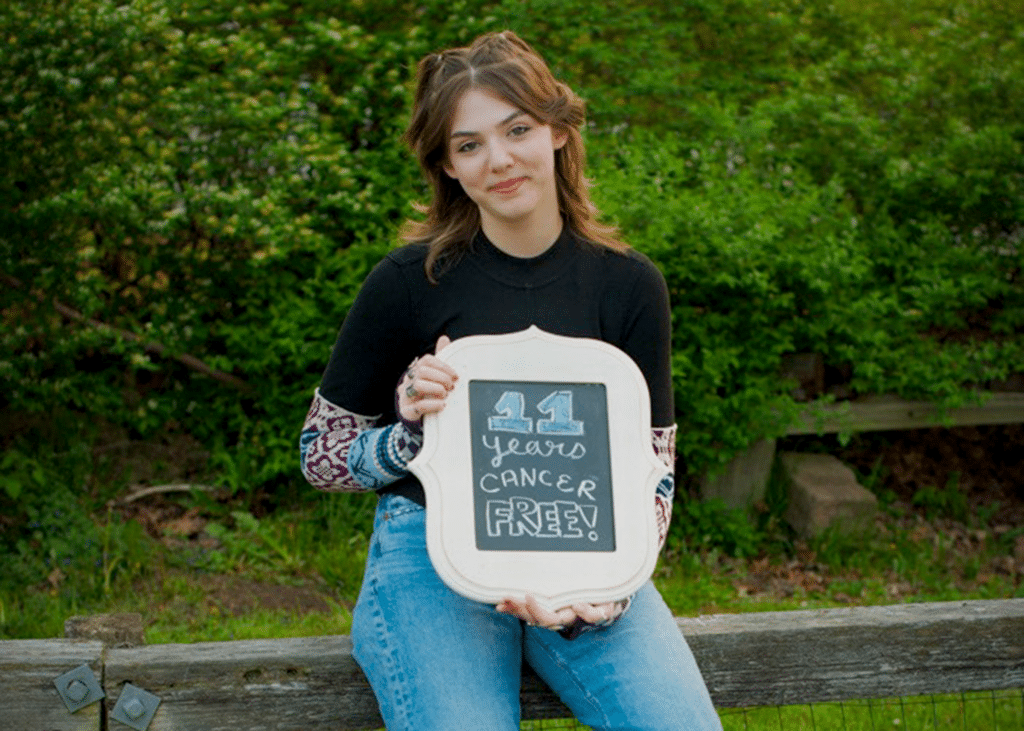
Which CAR-T products are currently on the market?
Since 2017, the approval of the world’s first CAR-T products, Novartis’s Kymriah, and Kite Pharma’s Yescarta by the FDA, has heralded a new era in cell immunotherapy. Presently, there are 10 CAR-T drugs approved for market globally. Additionally, there are over 1,000 CAR-T clinical trials registered worldwide on Clinicaltrials, with nearly 500 projects in mainland China. The primary treatment areas include hematologic malignancies, along with solid tumors such as pancreatic cancer, liver cancer, lung cancer, breast cancer, and colorectal cancer.
More and more people are choosing to seek medical treatment in China. China’s technology in oncology, especially in CAR-T, is now on par with that of the United States. The fundamental reasons why many individuals with related diseases choose China are the high-quality services, pleasant environment, and comparatively affordable prices.

“If you’d like to inquire about the latest cancer-fighting technologies and treatments, you can contact us.”
whatsapp:+8613717959070
