Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
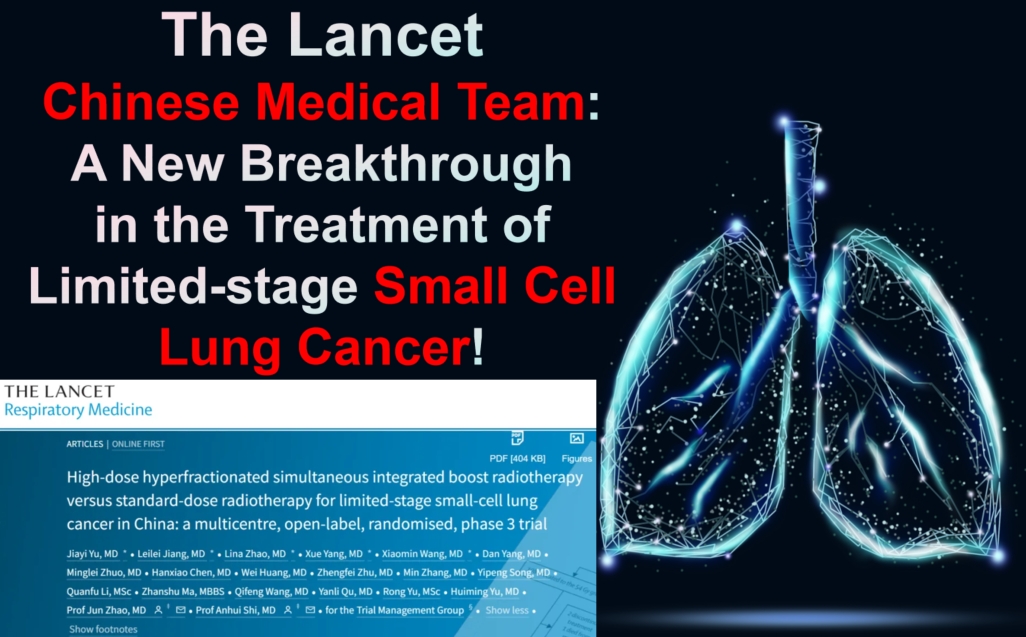
The Lancet | Chinese Medical Team: A New Breakthrough in the Treatment of Limited-stage Small Cell Lung Cancer!
The Lancet | Chinese Medical Team: A New Breakthrough in the Treatment of Limited-stage Small Cell Lung Cancer!
High-dose Accelerated Hyperfractionated Radiotherapy Helps Improve Treatment Efficacy

Lung Cancer
Small-cell lung cancer (SCLC) is a highly aggressive malignancy, accounting for 15% of all lung cancer cases. Among these, one-third are diagnosed with limited-stage SCLC (LS-SCLC), where the disease is confined to one side of the chest. For the past two decades, the standard treatment for LS-SCLC has been concurrent chemoradiotherapy, yet patients have seen little improvement in survival outcomes, with median survival ranging from 25-30 months.
A New Hope for LS-SCLC Patients
A recent study led by a team of Chinese researchers has shed light on a new treatment strategy. Published in The Lancet Respiratory Medicine, this multicenter, open-label, randomized phase III trial demonstrated that a high-dose, accelerated, hyperfractionated thoracic radiotherapy (54 Gy/30 fractions) combined with chemotherapy significantly improves overall survival (OS) and progression-free survival (PFS) for LS-SCLC patients. Importantly, this breakthrough treatment does not increase toxicity compared to the standard 45 Gy/30 fractions regimen.
Study Design and Methodology
The trial, conducted between 2017 and 2021, enrolled 224 patients across 16 hospitals in China. The patients, aged 18-70, had histologically or cytologically confirmed LS-SCLC and had either not yet received treatment or had undergone 1-2 cycles of chemotherapy. After chemotherapy, they were randomly assigned to receive either the high-dose (54 Gy/30 fractions) or standard-dose (45 Gy/30 fractions) thoracic radiotherapy, delivered twice daily over three weeks. Both groups also underwent four cycles of chemotherapy and, if responsive, prophylactic cranial irradiation (PCI) to prevent metastasis.
Improved Survival Outcomes
The study’s results were groundbreaking. Patients in the high-dose group showed a remarkable improvement in OS, with a median survival of 60.7 months, compared to 39.5 months in the standard-dose group. Additionally, the high-dose group saw a 45% reduction in the risk of death. Two-year survival rates also favored the high-dose group, with 76% of patients alive at two years, compared to 54% in the standard-dose group.
Progression-free survival was similarly improved, with the high-dose group experiencing a median PFS of 30.5 months, significantly longer than the 16.7 months observed in the standard-dose group. This translates to a 30% reduction in the risk of disease progression or death.
Safety and Tolerability
Despite the higher radiotherapy dose, the incidence of adverse effects between the two groups was comparable. The most common severe side effects were neutropenia, thrombocytopenia, and anemia, which occurred at similar rates in both groups. The occurrence of acute radiation-induced toxicity, such as esophagitis and radiation pneumonitis, also showed no significant differences. Importantly, there were no cases of severe late-stage toxicity, such as grade 3 esophageal stenosis or pulmonary fibrosis.
Clinical Implications and Future Directions
This study demonstrates that high-dose, accelerated hyperfractionated thoracic radiotherapy can significantly improve the prognosis of LS-SCLC patients without increasing treatment toxicity. It provides a new first-line treatment option that offers hope for longer survival. These findings may shift the clinical landscape, offering a viable alternative to the current standard of care.
As research continues to explore combining this high
A groundbreaking study led by a Chinese medical team has demonstrated that high-dose accelerated hyperfractionated chest radiotherapy (54 Gy/30 fractions) combined with chemotherapy significantly improves the overall survival (OS) of patients with limited-stage small-cell lung cancer (LS-SCLC). The research was published in The Lancet Respiratory Medicine on August 12, 2024. Compared to the standard dose of 45 Gy/30 fractions, the 54 Gy regimen increased OS without raising treatment toxicity, marking a potential shift in LS-SCLC treatment strategy.
Key Findings of the Study
LS-SCLC, a highly aggressive form of lung cancer, affects around 15% of all lung cancer patients, with one-third of them diagnosed at a limited stage. Traditionally, concurrent chemoradiotherapy (CRT) has been the preferred treatment option for LS-SCLC, yet the median survival rate has remained stagnant at around 25-30 months over the past two decades. The new study, however, highlights the efficacy of using higher doses of radiation to improve outcomes.
This phase III clinical trial was conducted across 16 hospitals in China between June 2017 and April 2021, involving 224 LS-SCLC patients. The trial compared two groups: a high-dose radiotherapy group (54 Gy/30 fractions twice daily) and a standard-dose group (45 Gy/30 fractions twice daily). Patients were treated with four cycles of chemotherapy and monitored through regular follow-ups. The results were remarkable.
Overall Survival: The high-dose group had a significantly extended median OS of 60.7 months compared to 39.5 months in the standard-dose group, reducing the risk of death by 45%.
Progression-Free Survival (PFS): The median PFS was also improved in the high-dose group, standing at 30.5 months compared to 16.7 months in the standard-dose group, with a 30% reduction in the risk of disease progression or death.
Safety: Despite the higher radiation dose, there was no increase in severe treatment-related side effects, such as neutropenia, thrombocytopenia, or radiation-induced pneumonia. Both groups exhibited similar rates of acute and long-term radiation toxicity.
Why Is This Study Important?
The study’s findings suggest that 54 Gy/30 fractions, delivered twice daily, may become a new standard of care for LS-SCLC patients. This approach significantly extends survival while maintaining a similar safety profile to the current standard dose. Moreover, with a two-year OS rate of 76% in the high-dose group, compared to 54% in the standard-dose group, the potential for long-term remission appears much greater.
A New Era in LS-SCLC Treatment?
While the current trial focused on radiotherapy and chemotherapy alone, future studies could explore combining this high-dose radiotherapy with immunotherapy. Recent breakthroughs, such as the ADRIATIC trial, have shown that immunotherapy following CRT can further improve survival outcomes for LS-SCLC patients. However, the safety of combining high-dose radiotherapy with immunotherapy needs further investigation due to potential risks like radiation-induced pneumonitis.
In conclusion, this study has paved the way for a new treatment option in LS-SCLC, offering hope for improved survival rates without additional toxicity. It marks a significant milestone in the battle against this aggressive form of lung cancer, providing a valuable alternative to the long-standing standard treatments.

 To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#LungCancerTreatment #SCLCTherapy #CancerResearch #MedicalBreakthrough #ChinaHealthcare #Oncology #LungCancerSurvival #Radiotherapy #CancerCare #ClinicalTrials
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
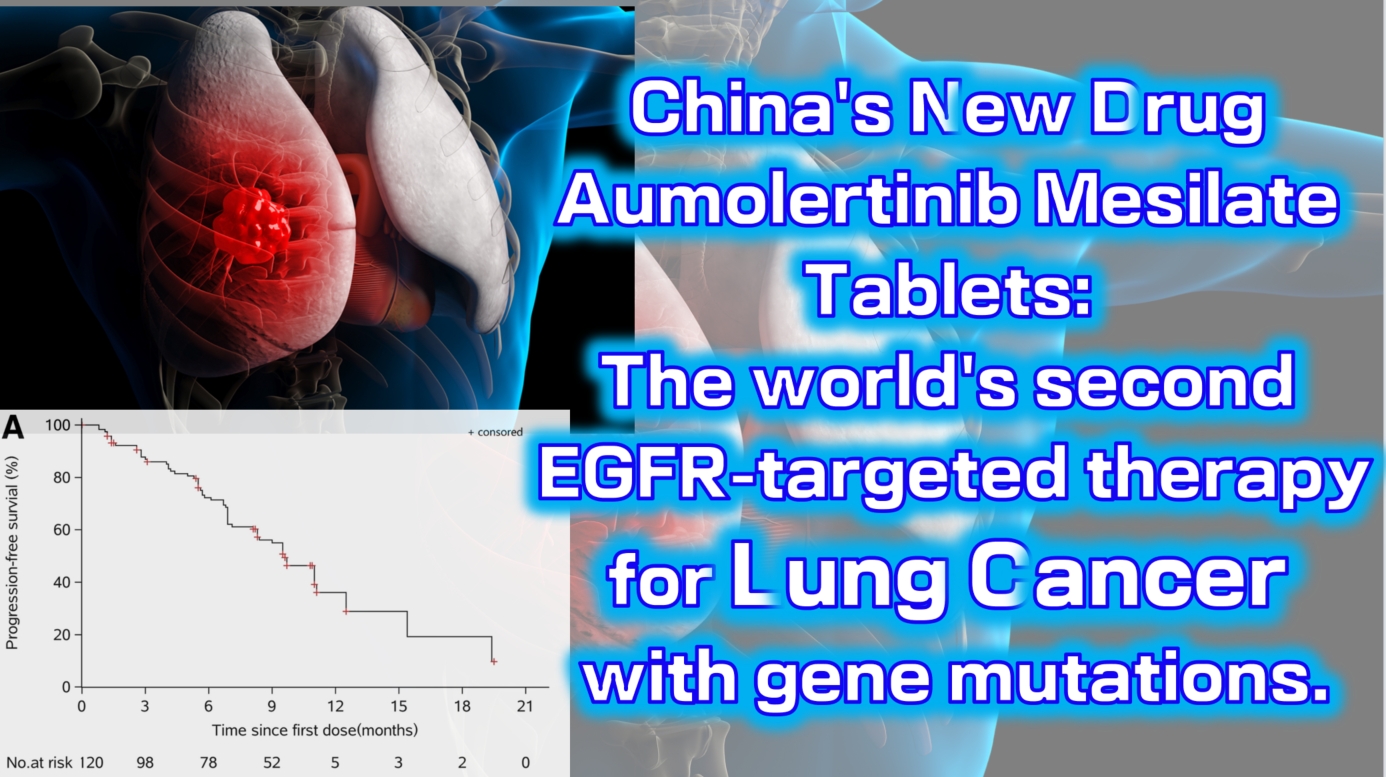
China’s new drug Aumolertinib Mesilate Tablets: The world’s second EGFR-targeted therapy for lung cancer with gene mutations.
China’s new drug Aumolertinib Mesilate Tablets: The world’s second EGFR-targeted therapy for lung cancer with gene mutations.

lung cancer
#LungCancer #EGFRInhibitor #TargetedTherapy #Aumolertinib #NSCLC
Aumolertinib Mesilate Tablets (Ameile) were approved for marketing in China on March 18, 2020. It is China’s first third-generation EGFR-targeted therapy for lung cancer with gene mutations and the second such drug globally, following Osimertinib.
On August 20, 2024, the new indication marketing application for Aumolertinib Mesilate Tablets was accepted for priority review by the China Center for Drug Evaluation (CDE). This indication is for the treatment of unresectable locally advanced non-small cell lung cancer (NSCLC) that has not progressed following platinum-based chemoradiotherapy. This is the fourth indication for which the drug has submitted a marketing application, and it is expected to be approved in the second quarter of 2025.
The Phase 1 clinical trial (NCT0298110) enrolled a total of 120 patients with locally advanced or metastatic non-small cell lung cancer (NSCLC). The results showed an objective response rate (ORR) of 52% (range: 42–63), with a disease control rate (DCR) as high as 92% (range: 84–96). The median progression-free survival was 11.0 months (95% CI: 9.5-not reached).

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#CancerTreatment #ClinicalTrials #PharmaInnovation #Oncology #DrugApproval #Biotech #PrecisionMedicine
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Hope to Eradicate Lung Cancer, Colorectal Cancer, and Various Solid Tumors! China’s First Self-Replicating mRNA Vaccine JCXH-211 Successfully Launched~
Hope to Eradicate Lung Cancer, Colorectal Cancer, and Various Solid Tumors! China’s First Self-Replicating mRNA Vaccine JCXH-211 Successfully Launched~

mRNA Vaccine
Tumor immunotherapy, as an emerging method for diagnosing and treating tumors, has garnered widespread attention since its inception. Prior to the advent of tumor immunotherapy, the clinical treatment of solid tumors relied solely on traditional surgical removal, radiotherapy, and chemotherapy. Although these methods were effective, they came with significant drawbacks such as drug side effects and the risk of recurrence.
Since the 1990s, mRNA vaccines have been considered therapeutic vaccines. During the COVID-19 pandemic in 2020, mRNA vaccines were used in clinical treatments, saving countless lives. Today, as the advantages of mRNA vaccines are gradually being discovered, they are now applied in multiple fields.
The advent of China’s first self-replicating mRNA vaccine JCXH-211 undoubtedly marks a new breakthrough in the clinical diagnosis and treatment of various solid tumors in China.
**What is the mRNA Vaccine JCXH-211?**
JCXH-211 is the world’s first self-replicating RNA drug encoding human interleukin-12 (IL-12) to enter clinical trials. This srRNA vaccine delivers mRNA encoding IL-12 into the cytoplasm, continuously expressing IL-12 to enhance the body’s immune response against tumor cells.
In preclinical studies involving various mouse and PDX disease models, JCXH-211 has shown the ability to effectively kill tumor cells, eliminate distant tumors, and prevent tumor recurrence. Comprehensive GLP toxicology studies have also demonstrated good safety.
JCXH-211 has a broad range of indications and is expected to effectively treat multiple solid tumors. The anticipated prospects are as follows:
**1. Lung Cancer:**
As one of the leading causes of cancer death worldwide, lung cancer is often diagnosed at a late stage due to difficulties in early detection. JCXH-211, through continuous expression of IL-12 and activation of immune cells, is expected to effectively control the growth and spread of lung cancer cells.
**2. Colorectal Cancer:**
Current clinical treatments for colorectal cancer primarily involve surgery and radiotherapy/chemotherapy, which can remove visible tumors but have a high recurrence rate. Periodic administration of JCXH-211 may significantly reduce recurrence and improve patient survival.
**3. Soft Tissue/Chondrosarcoma:**
These tumors are highly invasive and cover a wide range of areas, often affecting surrounding soft tissues and progressing rapidly. Current treatments are limited to surgery and radiotherapy/chemotherapy, which, although somewhat effective, are not thorough, leading to high recurrence rates. JCXH-211 could become a new treatment method, enhancing the body’s immune response to combat these stubborn cancer cells.
**4. Skin Cancer:**
Clinical treatments for skin cancer mainly include surgery and radiotherapy. While most skin cancers have a good prognosis, the effects on intractable melanoma are poor. The expression of IL-12 can activate specific T cells, greatly aiding in the elimination of melanoma cells.
**High-Efficiency Activation and Safety of the mRNA Vaccine JCXH-211!**
mRNA vaccines not only encode antigens to induce specific immune responses against tumors but also possess inherent immunostimulatory properties. This dual mechanism allows JCXH-211 to activate the immune system more efficiently, enhancing the overall anti-tumor effect.
Moreover, the safety of mRNA vaccines is well-recognized. Since DNA is the genetic material of most species, including humans and viruses, and is transcribed into mRNA before being translated into proteins to perform functions in the body, injecting mRNA into the human body does not enter the cell nucleus, thereby eliminating the risk of genomic insertion mutations. Additionally, mRNA can be naturally degraded and excreted from the body, preventing accumulation. Thus, JCXH-211 presents lower potential risks and is safer.
Currently, clinical trials are progressing steadily. With the rapid advancement of tumor immunotherapy, JCXH-211 is expected to become a standard treatment for various solid tumors, offering hope to many cancer patients.

 To assess whether the condition is suitable for cancer vaccine or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for cancer vaccine or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#LungCancer #ColorectalCancer #SolidTumors #mRNAVaccine #JCXH211 #CancerTreatment #TumorImmunotherapy #MedicalBreakthrough #Biopharma #CancerResearch #HealthcareInnovation #CancerHope #MedicalScience #HealthTech #ClinicalTrials #CancerSurvivor #CancerAwareness #InnovativeMedicine #CancerVaccine #Vaccine #mRNA #JCXH
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese Innovation in Medicine: A New Milestone in Small Cell Lung Cancer Treatment!

 **Chinese Innovation in Medicine: A New Milestone in Small Cell Lung Cancer Treatment!**
**Chinese Innovation in Medicine: A New Milestone in Small Cell Lung Cancer Treatment!**


Small Cell Lung Cancer
In the field of medicine, each new drug represents a leap forward in disease treatment, bringing new light and hope to patients. On May 9, 2024, the highly anticipated novel PD-L1 inhibitor, Bevosubab Single Monoclonal Antibody, officially received approval from the National Medical Products Administration (#NMPA) of China! This marks a vibrant addition to the treatment options for patients with extensive-stage small cell lung cancer, adding a brilliant rainbow to the sky of treatment.
Bevosubab Single Monoclonal Antibody is a Class I innovative drug declared by the Chinese biopharmaceutical subsidiary, Sinda Pharmaceuticals, for first-line treatment of extensive-stage small cell lung cancer.
Bevosubab Single Monoclonal Antibody is extraordinary—it is a brand-new PD-L1 inhibitor that directly targets the immune escape mechanism of tumor cells, awakening the body’s immune system to eradicate cancer cells invisibly. Combined with Anlotinib Hydrochloride, Etoposide, and Carboplatin, it forms a powerful treatment alliance, bringing new hope to small cell lung cancer.
This is not just a treatment regimen, but a redemption. In the ETER701 study, this four-drug combination therapy has rewritten the history of first-line treatment for small cell lung cancer! Survival has nearly doubled, greatly increasing the chances of survival and bringing more hope to patients.
What’s even more exciting is that, while using the combination therapy, the occurrence of adverse reactions is extremely manageable. This means that while gaining a longer life span, patients do not have to overly worry about the potential negative impacts of treatment.
The approval of Bevosubab Single Monoclonal Antibody not only opens new treatment doors for patients with extensive-stage small cell lung cancer but also brings new directions for exploration in the medical field. We are full of anticipation and believe that this new drug will shine brightly in future clinical practice, bringing endless hope and vitality to more patients!
To assess whether the condition is suitable for new durg therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <#AdvancedMedicineinChina> for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#SmallCellLungCancerTreatment #BevosubabSingleMonoclonalAntibody #NewDrugApproval #MedicalBreakthrough #cancer #cancertreatment #lung #lungcancer
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Introducing the Momentum of the Next-Generation “Pan-Cancer” Targeted Therapy ICP-723 of China
 Introducing the Momentum of the Next-Generation “Pan-Cancer” Targeted Therapy ICP-723 of China
Introducing the Momentum of the Next-Generation “Pan-Cancer” Targeted Therapy ICP-723 of China

Pan-Cancer
ICP-723
 On April 11, 2022, InnoCare Pharma unveiled the preclinical data of ICP-723. The research results demonstrate that ICP-723 effectively inhibits the kinase activity of TRKA, TRKB, and TRKC (IC50 value less than 1 nM). Not only does ICP-723 exhibit potent in vitro efficacy in TRK-driven tumors, but it also overcomes resistance mutations commonly seen after treatment with first-generation TRK inhibitors, such as TRKA G595R and TRKC G623R/E.
On April 11, 2022, InnoCare Pharma unveiled the preclinical data of ICP-723. The research results demonstrate that ICP-723 effectively inhibits the kinase activity of TRKA, TRKB, and TRKC (IC50 value less than 1 nM). Not only does ICP-723 exhibit potent in vitro efficacy in TRK-driven tumors, but it also overcomes resistance mutations commonly seen after treatment with first-generation TRK inhibitors, such as TRKA G595R and TRKC G623R/E.
TGI 89.5%
 Further in vivo efficacy studies have confirmed the robust anti-tumor effect of ICP-723 in animal models. At a dosage of 1 mg/kg, ICP-723 achieves a tumor growth inhibition rate (TGI) of 89.5% in the KM12 tumor model. With high oral bioavailability, ICP-723 demonstrates overall favorable pharmacokinetic parameters.
Further in vivo efficacy studies have confirmed the robust anti-tumor effect of ICP-723 in animal models. At a dosage of 1 mg/kg, ICP-723 achieves a tumor growth inhibition rate (TGI) of 89.5% in the KM12 tumor model. With high oral bioavailability, ICP-723 demonstrates overall favorable pharmacokinetic parameters.
Next-Generation NTRK Inhibitor
 ICP-723, a Chinese developed next-generation NTRK inhibitor, holds promise in treating advanced or metastatic solid tumors harboring NTRK fusion genes, including breast cancer, colorectal cancer, lung cancer, thyroid cancer, and patients resistant to first-generation NTRK inhibitors like larotrectinib and entrectinib.
ICP-723, a Chinese developed next-generation NTRK inhibitor, holds promise in treating advanced or metastatic solid tumors harboring NTRK fusion genes, including breast cancer, colorectal cancer, lung cancer, thyroid cancer, and patients resistant to first-generation NTRK inhibitors like larotrectinib and entrectinib.
 On August 31, 2021, InnoCare Pharma announced the Investigational New Drug (IND) approval from the U.S. Food and Drug Administration (FDA) to initiate Phase I clinical trials of its second-generation pan-TRK inhibitor ICP-723 in the United States.
On August 31, 2021, InnoCare Pharma announced the Investigational New Drug (IND) approval from the U.S. Food and Drug Administration (FDA) to initiate Phase I clinical trials of its second-generation pan-TRK inhibitor ICP-723 in the United States.
In the major financial performance report released by InnoCare Pharma in 2022, the efficacy of ICP-723 was also disclosed.
Objective Response Rate (ORR)
 As of February 11, 2022, in the Phase I dose-escalation trial, 17 patients received treatment with ICP-723 at doses ranging from 1 to 8 mg once daily. The safety profile was favorable, with no dose-limiting toxicities (DLT) observed. Among the 17 patients, 5 were confirmed positive for NTRK gene fusion, with an objective response rate (ORR) of 80%, including partial responses in 4 cases, and a disease control rate (DCR) of 100%. In the 4 mg and above dose group, the objective response rate was 100%.
As of February 11, 2022, in the Phase I dose-escalation trial, 17 patients received treatment with ICP-723 at doses ranging from 1 to 8 mg once daily. The safety profile was favorable, with no dose-limiting toxicities (DLT) observed. Among the 17 patients, 5 were confirmed positive for NTRK gene fusion, with an objective response rate (ORR) of 80%, including partial responses in 4 cases, and a disease control rate (DCR) of 100%. In the 4 mg and above dose group, the objective response rate was 100%.
 As a novel small molecule second-generation pan-TRK inhibitor, ICP-723 exhibits potent activity and high selectivity, offering the potential to overcome resistance to first-generation TRK inhibitors and better serve patients.
As a novel small molecule second-generation pan-TRK inhibitor, ICP-723 exhibits potent activity and high selectivity, offering the potential to overcome resistance to first-generation TRK inhibitors and better serve patients.
 (Note: This clinical trial is still recruiting participants. Patients who have undergone genetic testing can review their reports. Once NTRK1/2/3 fusion is detected, they can immediately contact us Advanced Medicine in China )
(Note: This clinical trial is still recruiting participants. Patients who have undergone genetic testing can review their reports. Once NTRK1/2/3 fusion is detected, they can immediately contact us Advanced Medicine in China )
 WhatsApp: +8613717959070
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
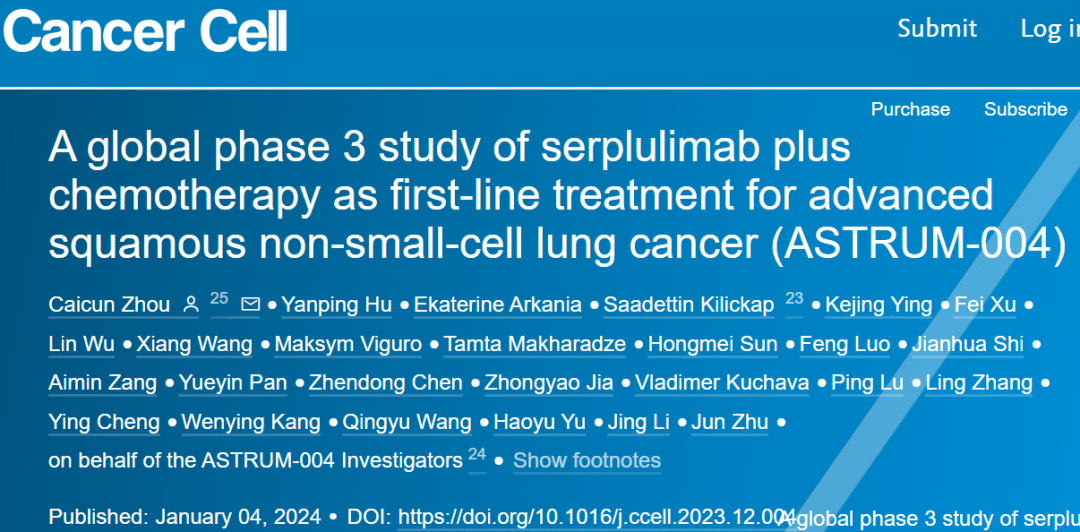
A New Era in Lung Cancer Treatment – Serplulimab Brings Innovative and Reliable Therapeutic Options!
**🌟 A New Era in Lung Cancer Treatment – Serplulimab Brings Innovative and Reliable Therapeutic Options! 🌟**

Cancer cell
📆CANCER CELL
January 5, 2023: The highly anticipated Phase 3 pivotal clinical trial (ASTRUM-004) of HANSIZHUANG, an anti-PD-1 monoclonal antibody developed independently by Henlius, in combination with chemotherapy for first-line treatment of advanced squamous non-small cell lung cancer (sqNSCLC), has just been published in the prestigious international oncology journal “Cancer Cell” (Impact Factor: 50.3)! Led by Professor Zhou Caicun from Shanghai Pulmonary Hospital, this milestone study signifies a significant breakthrough in lung cancer treatment.
🔬 Lung cancer
is one of the most common cancers globally, with the highest reported incidence and mortality rates in China. According to the latest statistics from the National Cancer Center, there are 828,000 new cases of lung cancer and 657,000 deaths annually. Non-small cell lung cancer (NSCLC) accounts for approximately 80% to 85% of all lung cancers, with a significant proportion diagnosed at an advanced stage or with metastasis, unsuitable for surgical resection.
🌱NSCLC
In the field of NSCLC treatment, immune checkpoint inhibitors have become a game-changer. Serplulimab is the first innovative monoclonal antibody developed independently by Henlius. Since its approval in March 2022, it has been approved in China for the treatment of microsatellite instability-high (MSI-H) solid tumors, sqNSCLC, extensive-stage small cell lung cancer (ES-SCLC), and esophageal squamous cell carcinoma (ESCC), benefiting over 51,000 patients.
🔍 ASTRUM-004
is a pivotal Phase 3 trial aimed at evaluating the efficacy and safety of serplulimab in combination with chemotherapy as first-line treatment for advanced sqNSCLC. The results are promising: patients receiving serplulimab demonstrated significantly prolonged progression-free survival (PFS) and overall survival (OS), with manageable safety profiles.
Serplulimab
🎉NEW ERA
These findings herald a new era in lung cancer treatment, offering a ray of hope for patients and clinicians alike. Serplulimab in combination with chemotherapy has emerged as a promising first-line treatment option, bringing innovation and reliability to sqNSCLC patients.

👩🔬Lead
Lead investigator Professor Zhou Caicun commented, “The ASTRUM-004 study validates the significant potential of serplulimab in combination with chemotherapy to improve survival outcomes in sqNSCLC patients, marking a major breakthrough in the field of lung cancer.”
🌟 Stay tuned for more updates as we continue to unravel the mysteries of cancer treatment, bringing hope to millions worldwide!
#CancerResearch #LungCancer #MedicalBreakthrough #InnovationForHealth #Serplulimab #NSCLC #ASTRUM #sqNSCLC #cancercell
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China’s Breakthrough: Sunvozertinib Leads the Wave of New Drug Development in EGFR exon20ins Mutant NSCLC
🌈🌈China’s Breakthrough: Sunvozertinib Leads the Wave of New Drug Development in EGFR exon20ins Mutant NSCLC🌸🌸
**Caption:**
🇨🇳 Exciting news from China’s research! Sunvozertinib, a novel drug by Dizal Pharmaceuticals, is making strides in treating EGFR exon20ins mutant NSCLC. 🌐✨

Sunvozertinib
**Article:**
Dizal Pharmaceuticals’ EGFR tyrosine kinase inhibitor (TKI) Sunvozertinib, known as 舒沃替尼 (Shuwo Tini) in China, has achieved a significant milestone with the publication of data from its Chinese registration study (WU-KONG6) in “The Lancet Respiratory Medicine.” This study validates the clinical efficacy of the Chinese-developed drug in treating late-stage non-small cell lung cancer (NSCLC) with EGFR exon20ins mutations, marking a crucial breakthrough for patients who have long faced challenges in finding effective treatments.
**Challenges in Treating EGFR exon20ins Mutant NSCLC**
EGFR exon20ins mutation has been a formidable subtype in EGFR-mutant NSCLC, with limited effectiveness observed in traditional EGFR-TKI and chemotherapy treatments. Faced with this challenge, pharmaceutical companies are actively engaged in new drug development to propel advancements in the treatment landscape for EGFR exon20ins mutant NSCLC. The success of the WU-KONG6 study signifies a critical recognition in this field and affirms the high efficacy and low toxicity of Sunvozertinib in treating EGFR exon20i

Lancet
ns mutant NSCLC.
**Results from the Chinese Registration Study “WU-KONG6″**
The results of the Chinese registration study “WU-KONG6” demonstrate that Sunvozertinib achieved an objective response rate (ORR) of 60.8% in treated patients with EGFR exon20ins mutations. This indicates a significant tumor reduction in over 60% of patients, leading to a substantial decrease in tumor burden. Moreover, patients experienced improvements in clinical symptoms and quality of life. Notably, Sunvozertinib is currently the only drug that has elevated the ORR in treated patients with EGFR exon20ins mutations to over 50%, breaking previous treatment ceilings and showcasing its potential as the “best in class.”
**Looking Ahead**
The competition in the field of EGFR exon20ins mutant NSCLC treatment is intensifying, and the successful introduction of Sunvozertinib provides a new therapeutic option for patients. As other pharmaceutical companies globally advance in new drug development, the innovation and competition in this field are expected to escalate, bringing more hope to patients with EGFR exon20ins mutant NSCLC. In the continual pursuit of medical breakthroughs, the emergence of new drugs will offer more effective treatment options, contributing to the extension of patients’ survival periods.
#MedicalInnovation #CancerResearch #HopeForPatients #Sunvozertinib #MedicalBreakthrough #ChinaInnovation
