Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese CAR-T Therapy: A New Frontier in Treating Systemic Lupus Erythematosus

Systemic Lupus Erythematosus
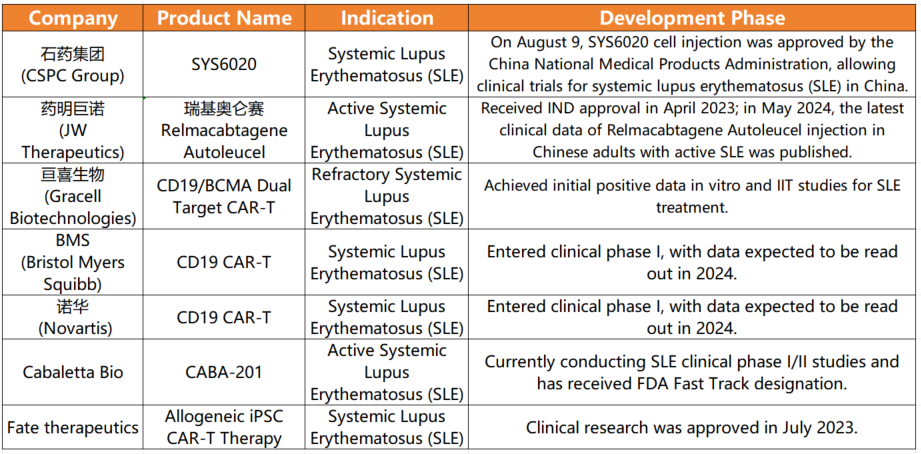
Recently, CSPC PHARMA’s CAR-T product, SYS6020, received approval for clinical trials from China’s National Medical Products Administration (NMPA). This groundbreaking development represents a significant milestone in cell therapy, especially for treating refractory active systemic lupus erythematosus (SLE). Notably, SYS6020 targets the BCMA antigen, potentially providing a novel and cost-effective treatment option for SLE patients worldwide.
**Innovative Approach and Promising Safety Profile**
SYS6020 is a pioneering mRNA-LNP-based CAR-T cell injection, marking it as the first of its kind to enter clinical trials. This innovative therapy specifically recognizes the BCMA antigen, attacking and eliminating immune cells responsible for elevated autoantibodies. This approach offers a unique, safe, and effective treatment option for SLE, distinct from traditional CAR-T therapies. Notably, SYS6020 exhibits high cell viability, a high CAR-positive rate, and reduced risks of genomic integration and cytokine release syndrome (CRS), common issues with conventional CAR-T treatments.
Preclinical studies have shown that SYS6020 effectively kills BCMA antigen-positive myeloma cells while maintaining a favorable safety profile. By employing LNP for T cell transfection, the therapy significantly reduces costs compared to using lentiviral vectors, lessening the financial burden on patients.
Lupus
**Revolutionizing Treatment for Autoimmune Diseases**
SLE, a complex chronic autoimmune disease, is characterized by the immune system attacking its own tissues. According to the “Chinese Guidelines for Diagnosis and Treatment of Lupus Nephritis,” the prevalence of SLE in China is estimated to be between 30.13 and 70.41 per 100,000 individuals, translating to 422,000 to 986,000 patients.
Traditional SLE treatments, including steroids, often come with severe side effects and long-term health risks. In contrast, biological therapies have emerged as a game-changer by modulating immune responses, reducing the immune system’s attack on the body’s tissues, and decreasing dependency on steroids. However, only three biologics—belimumab, tildrakizumab, and anifrolumab—are currently approved globally.
CAR-T therapy has shown remarkable efficacy in treating refractory SLE, providing hope in a challenging therapeutic landscape. This therapy precisely targets “rogue” B cells responsible for autoantibody production, thereby minimizing organ damage and offering a more targeted approach than traditional treatments.
**Future Prospects and Ongoing Challenges**
While CAR-T therapy presents a promising new direction for SLE treatment, its long-term efficacy and safety remain under investigation. Larger clinical trials are needed to validate its effectiveness and safety, as current studies are limited to small samples.
Additionally, the potential long-term risks of CAR-T therapy, such as increased susceptibility to infections or malignancies, require thorough exploration. Despite these uncertainties, the active involvement of multiple domestic and international cell therapy companies underscores the global commitment to advancing CAR-T therapies for autoimmune diseases.
In summary, CAR-T therapy for SLE holds immense potential, with clinical trials offering hope for a safer and more effective treatment alternative. Continued research and positive trial outcomes could establish CAR-T as a viable therapeutic option for SLE patients, revolutionizing their treatment landscape.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#CAR_Therapy #SLETreatment #AutoimmuneInnovation #MedicalBreakthrough #LupusAwareness



