Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese Research Team New Treatment Shows Promise: CD7 CAR-T Cell Therapy for Relapsed/Refractory AML
Chinese Research Team New Treatment Shows Promise: CD7 CAR-T Cell Therapy for Relapsed/Refractory AML

A recent clinical trial has unveiled a new prospect for treating relapsed/refractory acute myeloid leukemia (AML). The study delved into the safety and efficacy of a natural selection CD7 CAR-T cell (NS7 CAR-T cell) in treating CD7+ relapsed/refractory AML patients.
The research involved 10 cases of CD7+ relapsed/refractory AML, where 30% of AML patients expressed CD7. Following treatment with NS7 CAR-T cell infusion, the results showed that within 4 weeks post-infusion, 70% of patients achieved complete bone marrow remission (CR), with 6 patients achieving a deep remission at the level of minimal residual disease (MRD). Notably, the loss of CD7 was identified as a primary reason for non-response in some patients.
The study also indicated that the majority of patients (80%) experienced mild cytokine release syndrome (CRS) post-infusion, with no observed neurotoxicity.
Furthermore, for select patients, continuing with a second allogeneic hematopoietic stem cell transplantation post NS7 CAR-T cell treatment resulted in promising long-term outcomes. However, further exploration is required for patients who did not undergo consolidative transplants to enhance treatment efficacy.
Professor Lu Peihua(Lu Daopei Hospital) remarked that this study highlights the potential of CD7 CAR-T cells as a bridging therapy before transplantation, demonstrating preliminary therapeutic efficacy for relapsed/refractory AML patients. Nevertheless, a comprehensive evaluation of its efficacy requires a larger sample size and longer-term follow-up.

This preliminary study represents a significant advancement in the field of AML treatment, but collaborative efforts and more research are still necessary. Looking ahead, we anticipate further studies and treatment outcomes to bring more beneficial therapeutic options for the long-term survival of patients, supported by a broader patient cohort and collaborative efforts from the medical community.
The outlook for this CD7 CAR-T cell therapy is promising, especially for AML patients who have undergone various treatments and allogeneic hematopoietic stem cell transplants. We look forward to more in-depth research and treatment outcomes in the future, offering more beneficial treatment choices for long-term survival among patients.
(source: ClinicalTrials.gov registration number NCT04938115
Preliminary results published by Professor Lu Peihua and their team in a top-tier journal)

#AMLResearch #CancerTreatment #CARTTherapy #Leukemia #MedicalBreakthrough #Cancer #AML #Immunotherapy #ClinicalTrials #BloodCancer #PatientCare #HealthcareAdvances #PrecisionMedicine #ScienceAndHealth #MedicalScience #CancerSurvivor #AMLWarriors #ResearchProgress #CancerFree #HopeInTreatment #acutemyeloidleukemia
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
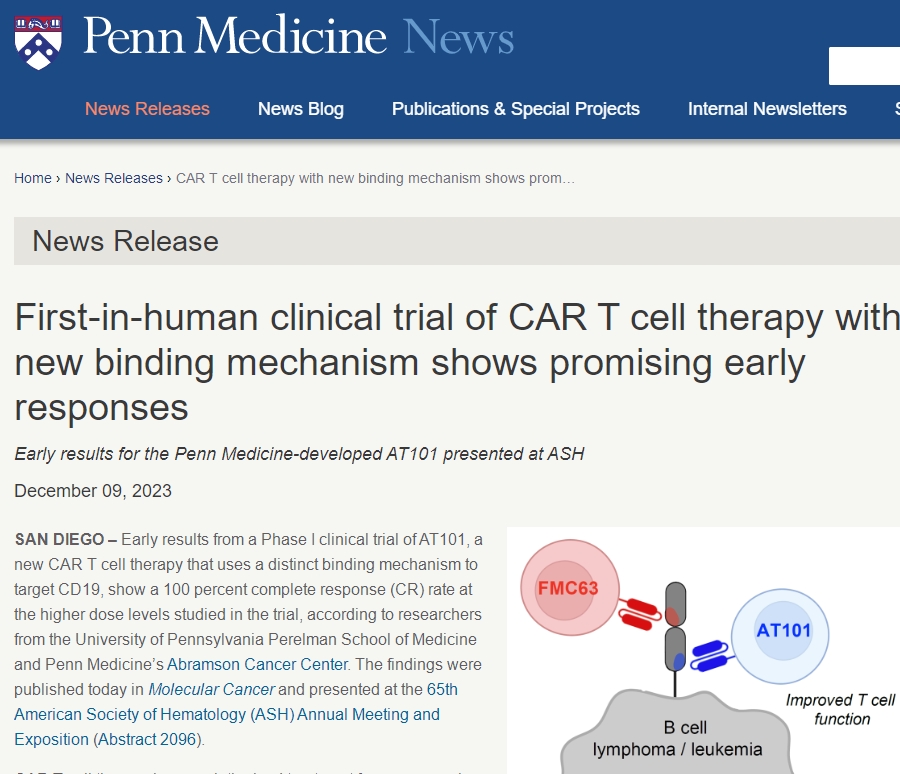
New Generation CAR-T Cell Therapy AT101: Phase I Clinical Trial Shows 100% Complete Remission Rate!
New Generation CAR-T Cell Therapy AT101: Phase I Clinical Trial Shows 100% Complete Remission Rate!

Researchers recently released exciting news: the Phase I clinical trial of their newly developed CAR-T cell therapy, AT101, demonstrated a 100% complete remission rate among patients receiving high-dose treatment. This groundbreaking achievement was published in the latest “Molecular Cancer” journal and gained significant attention at the 65th American Society of Hematology (ASH) Annual Meeting.
Despite recent FDA investigations into the safety of CAR-T cell therapy, it remains the most promising choice for blood cancer patients who have tried other treatment methods unsuccessfully. CAR-T cell therapy has fundamentally changed the treatment landscape for many blood cancer patients. While some patients show long-term responses to it, others do not.
The new CAR-T cell therapy, AT101, developed by researchers from the Perelman School of Medicine and the Abramson Cancer Center at the University of Pennsylvania, exhibited highly encouraging results due to its design targeting a new epitope of CD19 through a unique binding mechanism. Most currently approved CD19 CAR-T cell therapies target the same epitope, yet many patients eventually relapse. AT101, by targeting a different CD19 epitope, shows faster action rates and aims to reduce the failure rate of CAR-T cell therapy while improving clinical efficacy.
In this Phase I clinical trial, 14 patients with relapsed or refractory B-cell non-Hodgkin lymphoma (NHL) received treatment. Nine out of twelve patients achieved a complete remission status, indicating a total remission rate of 91.7%, with eight patients achieving complete remission. These patients did not experience cancer relapse, and the drug showed promising safety results.
While further research and larger-scale clinical trials are necessary, these early results instill considerable confidence. The success of AT101 holds promise for bringing new hope to blood cancer patients, especially those who previously did not receive effective CAR-T cell therapy. This study also aims to expand to a broader patient population, offering more treatment options and possibilities.
#CARTTherapy #BloodCancerTreatment #AT101Research #CancerRemission #MedicalBreakthrough #ASHAnnualMeeting #ClinicalTrialSuccess #UniversityResearch #CancerResearchUpdate #HopeForPatients #AT101 #BloodCancer #CancerTreatment
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

A significant breakthrough in Chinese solid tumor CAR-T therapy, resulting in complete remission for pancreatic cancer patients.
Chinese pharmaceutical company SciCoMed announced a significant breakthrough in treating metastatic pancreatic cancer with its independently developed CAR-T cell therapy targeting Claudin18.2, known as CT041. Pancreatic cancer, often termed the “king of cancers,” presents a significant challenge with low survival rates. However, CT041’s two representative cases have garnered attention: two patients with refractory pancreatic cancer, who failed multiple lines of treatment, received CT041 therapy. The results showcased a substantial reduction in tumor lesions and, in some cases, complete disappearance, offering a glimmer of hope for pancreatic cancer patients.

In the case of a 58-year-old female patient, lung metastases significantly decreased post CT041 treatment. Similarly, a 75-year-old female patient achieved complete remission by the fourth week post-treatment, maintaining remission to date. These successful cases validate the remarkable efficacy of CT041 for refractory pancreatic cancer, sparking interest and anticipation among international experts.
Moreover, CT041 has garnered recognition for its efficacy in the field of digestive system tumors. As the world’s first CAR-T cell therapy targeting Claudin18.2, it demonstrates promising prospects in treating digestive system tumors. Research data illustrates that among 37 patients, 83.3% experienced tumor regression, with an objective response rate of 48.6%. Additionally, CT041 displayed significant effectiveness in late-stage gastric and pancreatic cancer patients, offering hope for future treatments.
The rapid development of CAR-T therapy in China involves over 20 companies contributing significantly. CT041’s success signifies China’s progress toward becoming a global leader in CAR-T therapy. Moving forward, scientists will continue efforts to enhance efficacy and reduce side effects, providing a ray of hope for more late-stage cancer patients.
