Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
Unlocking the Potential: Understanding TIL Therapy for Solid Tumors
🔍 Unlocking the Potential: Understanding TIL Therapy for Solid Tumors🧬
💡What is TIL therapy?🧬
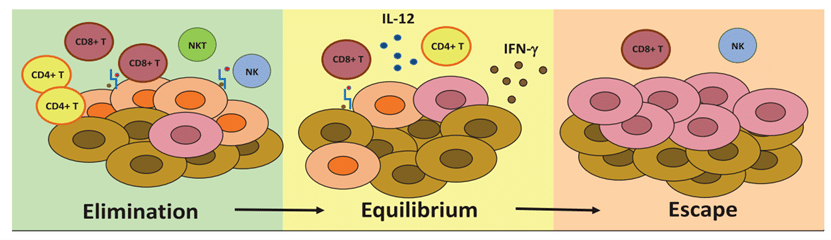
Cancer Development
Understanding Cancer Development: Immune Surveillance, Immune Equilibrium, and Immune Escape ���

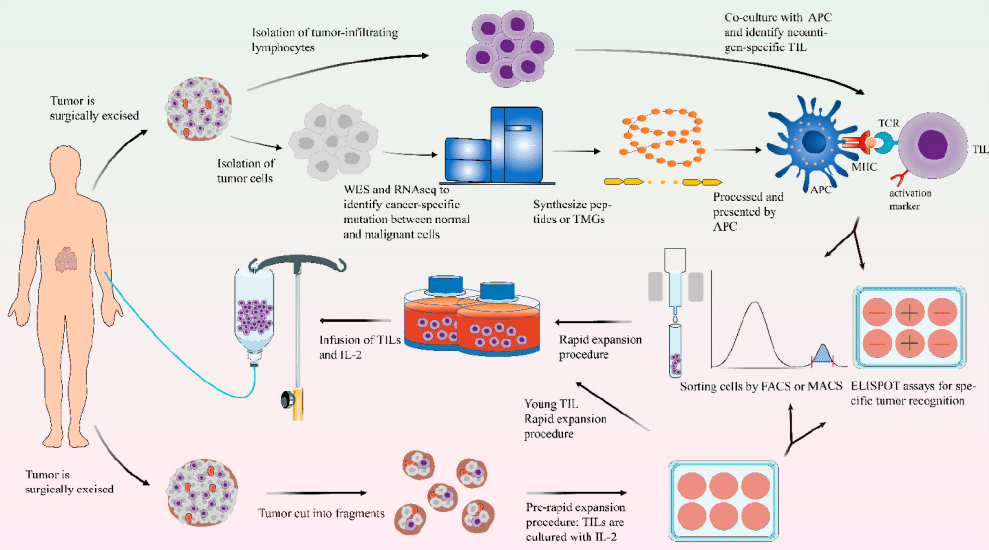
TIL Therapy
🔬 The main routine steps of TIL therapy include:
💪Characteristics of TIL therapy:
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Exciting News Alert! China’s New TCR-T Product IND Approved for Synovial Sarcoma!
🌟 Exciting News Alert! China’s New TCR-T Product IND Approved for Synovial Sarcoma! 🌟

synovial sarcoma

Cell Rep Med
China’s first TCR-T product IND approval takes aim at synovial sarcoma.
TAEST16001,

TCR-T Therapy
How to Seek TCR-T Therapy Assistance
or click on the WhatsApp+8613717959070
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China’s Breakthrough: Sunvozertinib Leads the Wave of New Drug Development in EGFR exon20ins Mutant NSCLC
🌈🌈China’s Breakthrough: Sunvozertinib Leads the Wave of New Drug Development in EGFR exon20ins Mutant NSCLC🌸🌸
**Caption:**
🇨🇳 Exciting news from China’s research! Sunvozertinib, a novel drug by Dizal Pharmaceuticals, is making strides in treating EGFR exon20ins mutant NSCLC. 🌐✨

Sunvozertinib
**Article:**
**Challenges in Treating EGFR exon20ins Mutant NSCLC**
Lancet
ns mutant NSCLC.
**Results from the Chinese Registration Study “WU-KONG6″**
**Looking Ahead**
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
2024 Lancet: Revolutionizing Cancer Treatment: China’s Breakthroughs in CAR T-Cell Therapy
🌟2024 Lancet: Revolutionizing Cancer Treatment: China’s Breakthroughs in CAR T-Cell Therapy 🌏

LANCET
Dive into the cutting-edge world of CAR T-cell therapy, where China is making waves in the realm of cellular treatments. Since the inception of CAR T-cell clinical trials in 2013, this groundbreaking therapy has become a beacon of hope for cancer patients across the country.
🔬 Explosive Growth: By 2017, China led the globe in the number of CAR T-cell-related clinical trials, marking a pivotal moment in the evolution of cancer treatments. Fast forward to 2021, and Chinese cell therapy companies have amassed a staggering $237 million in funding, reflecting the robust expansion of CAR T-cell therapy in the nation.
🌐 Government Support: A deep dive into the research unveils the influential role of Chinese government policies in propelling CAR T-cell therapy forward. Strong governmental backing, coupled with capital influx, massive patient demand, and a unique healthcare system, lays the foundation for the accelerated growth of this revolutionary therapy in China.
🎗️ Overcoming Challenges: While CAR T-cell therapy is still in its infancy for solid tumors, it has achieved remarkable success in treating blood cancers. China has emerged as a global leader in conducting clinical trials, particularly focusing on hematologic malignancies like B-cell lymphomas. CAR-T cells, reprogrammed to combat cancer, offer a beacon of hope for those facing these formidable diseases.
🚀 Pushing Boundaries: Beyond blood cancers, Chinese researchers are actively exploring the potential of CAR-T cell therapy in various solid tumors. Preliminary studies hint at promising results for liver, pancreatic, and brain cancers. China’s commitment to medical innovation shines through as it pushes the boundaries of cancer research and treatment strategies.
🌈 Hope for the Future: Join us in celebrating the strides China is making in cancer research. Every breakthrough in CAR T-cell therapy brings us closer to a future where cancer is not just treated but conquered.
💪🏼🔬 #CARTCellChina #MedicalInnovation #CancerBreakthrough #HopeForTheFuture #GlobalHealthRevolution #CellTherapy #LANCET #HEMATOLOGY
The Emergence of Fifth Generation CAR-T: A Boon for Late-Stage Cancer Patients or a Major Breakthrough in Solid Tumor Treatment?
The fifth-generation CAR-T is designed as a universal type of CAR-T. Is this risk-free CAR-T capable of achieving significant breakthroughs in solid tumor treatment, or is it effectively reducing costs to enable scalable production and treatment?
After nearly three decades of development, CAR (Chimeric Antigen Receptor) technology has undergone continuous innovation. Currently, CAR has evolved to its fifth generation. Its aim is to enhance the safety of treatments by reducing toxicity and non-specific antigen recognition. This is achieved by stimulating proliferation, activation, and the generation of memory phenotypes within CAR-T cells to improve efficiency and provide immune regulation for the optimal function of CAR-T cells.
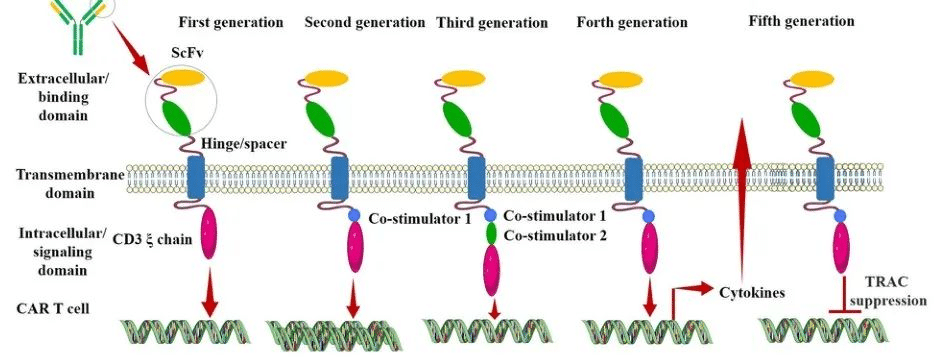
Generation CAR-T
The Evolution of Different Generations of CAR-T
First Generation CAR:
The first-generation CAR comprises an extracellular single-chain variable fragment (scFv) as the antigen recognition binding domain and an intracellular CD3ζ as the cellular activation signaling domain. Despite initiating cytotoxic anti-tumor responses within transplanted T cells, first-generation CAR-T cells exhibit lower levels of cytotoxicity and proliferation due to the CAR structure lacking co-stimulatory domains, which results in inadequate interleukin (IL)-2 production.
Second Generation CAR:
Building upon the CD3ζ signal transduction domain, the second-generation CAR includes an additional co-stimulatory signaling domain that activates T cells, significantly enhancing T cell proliferation and survival. For instance, CD28 can deliver robust activation signals, enabling T cells to achieve high levels of cytotoxic activity in a shorter duration, while 4-1BB provides prolonged activation signals, sustaining T cell-mediated killing of tumor cells. However, limitations arise in second-generation CAR-T cells utilizing retroviruses as viral vectors, restricting the length of transgene fragments they can carry. As a result, it becomes necessary to choose between incorporating CD28 and 4-1BB into T lymphocytes.
Third Generation CAR:
Third-generation CAR-T cells utilize larger DNA-carrying lentiviruses as viral vectors, allowing simultaneous incorporation of DNA fragments for both CD28 and 4-1BB into T cells. Consequently, the third-generation CAR structure encompasses two co-stimulatory domains, theoretically addressing the need for higher activation intensity and sustained survival of CAR-T cells. However, the safety concerns associated with prolonged and high-level persistence of CAR-T cells, including potential attacks on the host’s immune system, remain unresolved despite these advancements.
Fourth Generation CAR:
The design concept behind the fourth-generation CAR revolves around the precise treatment of cancerous diseases. For instance, solid tumors generate a microenvironment (TME) during their chronic progression, preventing CAR-T cells from penetrating the tumor interior. As a result, CAR-T therapy demonstrates limited efficacy in treating solid tumors. TRUCK CAR-T involves incorporating cytokines (such as IL-12) or chemokines into the CAR structure. This facilitates increased infiltration of T cells into tumor tissues while recruiting other immune cells within the body to eliminate tumor cells. In some studies, a suicide gene or certain drug-sensitive genes are attached to the CAR structure to ensure the clearance of CAR-T cells from the body post-treatment, preventing inadvertent harm to normal cells and enhancing the safety and controllability of CAR-T therapy.
Fifth Generation CAR:
The fifth-generation CAR-T, known as universal CAR-T, achieves T-cell receptor α (TCR-α) and β (TCR-β) chain deletion by knocking out the TRAC gene. This implies the removal of the T-cell receptor (TCR) from the surface of T cells, thereby avoiding the occurrence of graft-versus-host disease (GVHD) in transplantation reactions.
Since the FDA’s approval of the CD19 CAR-T product, Novartis’s Kymriah, in 2017, CAR-T cell therapy has entered a stage of rapid development. However, the currently approved and marketed products are all second-generation CAR-T therapies. There is still a long way to go for CAR-T to become widespread in the market.
Safety concerns constitute the primary challenge for CAR-T, such as off-target effects, cytokine release syndrome (CRS), and neurotoxicity (NTX). Currently available CAR-T products primarily focus on treating hematologic malignancies, with no major breakthroughs achieved yet in treating solid tumors.
In 2021, China’s NMPA approved three CAR-T products for marketing: FOSUNKITE’s Axicabtagene Ciloleucel injection, JW Therapeutics’s Relmacabtagene Autoleucel Injection, and the recently approved JUVENTAS’s Inaticabtagene Autoleucel Injection, all targeting CD19. Additionally, earlier this year, IASO Bio obtained approval for Equecabtagene Autoleucel Injection, targeting BCMA. While CAR-T targeting CD19 has shown effectiveness, its scope remains limited to B-cell-related hematologic malignancies. BCMA-targeted CAR-T is restricted to treating multiple myeloma. To address solid tumor treatment, the development of more specific and potent targets is necessary.
Among the recently released domestically developed JUVENTAS’s Inaticabtagene Autoleucel Injection, its competitive advantage lies in its price, which has decreased to below one million RMB(Approximately $140,000 US).
With the continuous advancement of molecular biology technologies, more breakthroughs are expected in CAR molecule design. This progression anticipates the development of safer and more efficient universal CAR-T therapies in the future, benefiting a broader spectrum of cancer patients.
“If you’d like to inquire about the latest cancer-fighting technologies and treatments, you can contact us.”
whatsapp:+8613717959070
#CARTCellTherapy #CancerTreatment #ScienceInnovation #GeneticMedicine #TumorTreatment #HealthcareTech #MedicalScience #CancerAwareness #PatientCare #FutureOfMedicine
