Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

A New Chapter in Survival! A Quadruple Drug Combination Opens Up a New Treatment Pathway for Multiple Myeloma Patients
🌟 *A New Chapter in Survival! A Quadruple Drug Combination Opens Up a New Treatment Pathway for Multiple Myeloma Patients.* 🌟

#NEJM, #Multiple myeloma
🔬 **Research Findings**
📊 **Study Results**
💡 **Conclusion**
🔍 **Learn More**
doctor.huang@globecancer.com
WhatsApp+8613717959070
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese CAR-T cell clinical and therapeutic have entered a period of rapid growth!
![]()
![]() **Chinese CAR-T cell clinical and therapeutic have entered a period of rapid growth!**
**Chinese CAR-T cell clinical and therapeutic have entered a period of rapid growth!** ![]()
![]()

Chinese CAR-T
According to the American clinical trial database Clinicaltrials, as of November 10, 2023, China has taken the lead in CAR-T cell therapy clinical research with a total of 655 studies, marking the country’s first foray into the international forefront of a new drug development field. Among them, Legend Biotech’s SicajioLunsei and Koji Pharmaceutical’s Claudin18.2 CAR-T have both achieved global leadership. Additionally, several companies are also advancing next-generation CAR-T and allogeneic CAR-T technologies, showing promising initial data and potential for global market success.

CAR-T therapy
“If you’d like to inquire about the latest cancer-fighting technologies and treatments, you can contact us.”
whatsapp: 137 1795 9070
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Exciting breakthrough in Chinese pharmaceutical research!
[🔬🌏] Exciting breakthrough in Chinese pharmaceutical research!

Chinese medicial research
💊Since 2019, China has witnessed a surge in phase 1 and 2 clinical trials, with a focus on addressing prevalent cancers like stomach, esophageal, and cervical cancers. The world is now turning its attention to China’s rapid emergence on the global stage of pharmaceutical innovation.
🌟 Key Highlights:
🚀 2020 and Beyond:
🌿 Traditional Medicine Meets Innovation:
🔍 Future of Cellular Therapy:
Breakthrough medical research in China brings hope for multiple myeloma patients.
Eque-cel brings new treatment prospects for multiple myeloma.
From December 9th to 12th, the Annual American Society of Hematology Meeting (ASH 2023) was held in San Diego, USA. At this conference, the FUMANBA-1 study, led by Professors Chunrui Li from Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, and Lugui Qiu from the Institute of Hematology, Chinese Academy of Medical Sciences, conducted at 14 medical centers nationwide, demonstrated the efficacy and characteristics of sustained minimal residual disease (MRD) negativity in multiple myeloma (MM) patients treated with Eque-cel injection (CT103A). Their achievements were included in the oral presentation at the conference.

Current MM Treatment and Challenges of CAR-T Therapy CAR-T cell therapy holds significant importance in the treatment of multiple myeloma, overcoming patients’ immunodeficiency and tolerance issues. Currently, China has approved three CAR-T cell drugs targeting multiple myeloma. Compared to traditional treatments, CAR-T cell therapy enhances immune function without relying on the major histocompatibility complex (MHC), improving the activity of NK cells, T cells, and B cells while effectively activating T cells. However, despite significant advancements in CAR-T cell therapy in the MM field, some patients still struggle to maintain treatment effectiveness, leading to potential relapses, with antigen escape being one of the important mechanisms causing relapse.
Furthermore, the safety of CAR-T therapy is a major concern, including adverse reactions such as cytokine release syndrome (CRS), neurotoxicity, infections, B cell depletion, and hypogammaglobulinemia. Hence, monitoring patient indicators throughout the entire CAR-T treatment process, from pre-treatment to infusion, is crucial for timely intervention in adverse reactions.
100% MRD Negativity Rate for CR and Above Patients! Eque-cel Injection Brings Redemption for Multiple Myeloma
Eque-cel injection (CT103A) is a fully humanized CAR-T cell therapy drug targeting B cell maturation antigen (BCMA), approved by the National Medical Products Administration (NMPA) for adult refractory/relapsed multiple myeloma patients in China. In the Phase II FUMANBA-1 study, this treatment showed significant and sustained efficacy.
As of December 31, 2022, with a median follow-up time of 18.07 months, deep and sustained remissions were observed in 103 evaluable patients. The overall response rate (ORR) among these 103 patients was 96.1%, with a strict complete response (sCR)/complete response (CR) rate of 77.7%. Among 91 subjects with no previous CAR-T treatment history, the ORR was 98.9%, and the sCR/CR rate reached 82.4%, with a 12-month progression-free survival (PFS) rate of 85.5%.
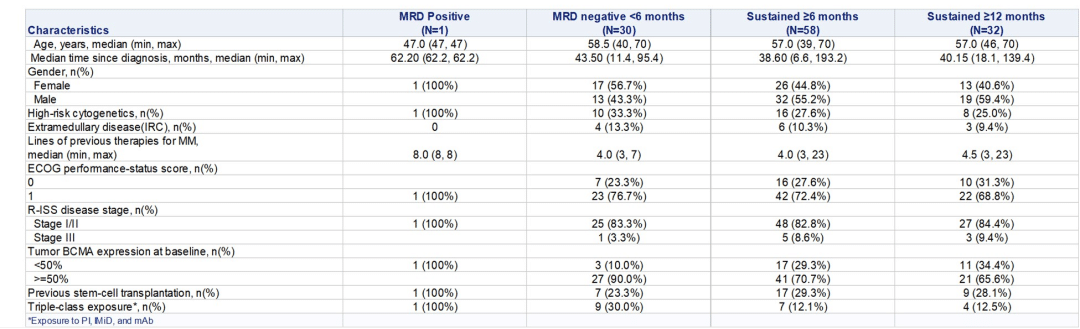
The minimal residual disease (MRD) negativity rate for the entire population was 94.2%, reaching 100% for CR and above patients. The median time to MRD negativity was 15 days, and 80.8% of patients maintained MRD negativity 12 months post-infusion. Moreover, the Eque-cel injection exhibited a relatively long median persistence in the body, lasting up to 307.5 days.
According to Professor Chunrui Li, “The sustained MRD-negative status is closely related to the improvement in PFS of patients receiving Eque-cel treatment.”
Professor Chunrui Li’s team studied the characteristics of patients maintaining MRD negativity for ≥6 months and ≥12 months in the FUMANBA-1 study. The research revealed that among 88 patients achieving MRD negativity, 78.4% maintained MRD negativity for at least 6 months, and 74.4% sustained MRD negativity for at least 12 months. Patients with sustained MRD negativity showed longer…

Professor Chunrui Li:
Chief Physician, Doctoral Supervisor
Secretary of the Hematology Department Party Committee, Deputy Director of Medicine Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology Professional
Focus: Immunotherapy for Hematological Malignancies
Member of the 11th Committee of the Plasma Cell Disease Professional Group of the Hematology Branch of the Chinese Medical Association
Chairman of the Expert Committee on Hematology (Hubei), Geriatrics Branch, Chinese Geriatrics and Gerontology Society
Standing Committee Member of the Geriatric Medicine Branch, Chinese Geriatrics and Gerontology Society
Youth Committee Member of the Oncology Branch, Chinese Anti-Cancer Association Committee Member of the Myeloma and Plasma Cell Disease Professional Group, 5th Committee of the Chinese Society of Clinical Oncology (CSCO) Leukemia Alliance & Lymphoma Alliance
Vice Chairman of the Blood Branch of the Hubei Medical Immunology Association Principal Investigator of four National Natural Science Foundation projects; published more than 20 SCI papers as first author or corresponding author, including Blood.
#EqueCelTreatment #MMResearch #CARTTherapy #MRDNegativity #CancerTreatment #MedicalBreakthrough #FUMANBA1Study #Immunotherapy #CancerResearchUpdate #CancerResearch #ASH2023 #BloodCancer #EqueCel #MRD #MultipleMyeloma
