Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

New Advances in Chinese CAR-T Cell Therapy: CD27 Armored BCMA CAR-T Shows Potential in Treating Relapsed Multiple Myeloma
**New Advances in Chinese CAR-T Cell Therapy: CD27 Armored BCMA CAR-T Shows Potential in Treating Relapsed Multiple Myeloma**

Multiple Myeloma
#BCMA #CD27#CARTCellTherapy #RRMM #MM #MultipleMyeloma #CART
Relapsed and refractory multiple myeloma (RRMM) is a challenging blood cancer that poses significant obstacles for patients. Although several BCMA (B-cell maturation antigen) targeting CAR-T cell therapies have been developed, achieving long-term remission and extended survival remains difficult. The recent introduction of CD27-armored BCMA CAR-T—CBG-002—by a Chinese medical team brings new hope for RRMM patients. Based on preclinical data, this therapy shows promising anti-myeloma activity, with Phase I clinical trial results further validating its efficacy and safety.
Research Highlights
CBG-002 is an innovative therapy that enhances traditional BCMA CAR-T by adding a CD27 costimulatory domain. The inclusion of CD27 improves CAR-T cell persistence and activity, contributing to stronger anti-cancer effects. Recently published Phase I research in Cancer Immunology Research indicates that this CD27-armored BCMA CAR-T therapy is not only well-tolerated but also shows notable efficacy in RRMM patients.
The study enrolled 11 RRMM patients, all of whom had undergone three or more prior treatments, with a median age of 54. The results showed that 81.8% of patients experienced only mild cytokine release syndrome (CRS) and no severe neurotoxicity. Even at a low dosage level (1-3×10^6 CAR-T cells/kg), CBG-002 achieved a high overall response rate (ORR) of 81.8%, with 45.5% of patients reaching stringent complete remission.
Future Outlook
The Phase I study of CBG-002 not only confirmed its efficacy but also demonstrated advantages in production time and cost. Compared to current products on the market, CBG-002 has a shortened production cycle of just 10 days, making it a timely and affordable treatment option for more patients. Amid global progress in CAR-T cell therapies, the innovation by this Chinese medical team positions it as a significant force in advancing cancer research worldwide.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#MultipleMyeloma #CancerResearch #Immunotherapy #ChinaBiotech #Oncology
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

What Kind of Multiple Myeloma Patients Are Suitable for CAR-T Therapy in China?
# What Kind of Multiple Myeloma Patients Are Suitable for CAR-T Therapy in China?

Multiple Myeloma
#MultipleMyeloma #CAR_Therapy #MM #CART #RRMM #Hematology
**Multiple Myeloma** (MM) is a malignant hematological disease that affects plasma cells. In recent years, various drugs and treatment methods, such as proteasome inhibitors, immunomodulatory drugs, and autologous stem cell transplantation, have significantly improved the prognosis of multiple myeloma patients. However, a considerable number of patients still experience relapses and refractory disease. For these patients, CAR-T cell therapy is emerging as a groundbreaking and effective treatment option.
China has made remarkable progress in CAR-T therapy technology in recent years, becoming a global leader in the field of hematological diseases. This has attracted patients worldwide to seek this advanced treatment. So, which multiple myeloma patients are suitable to receive CAR-T treatment in China?
## 1. **Indications: Preferred Choice for Drug-Resistant/Relapsed/Refractory Multiple Myeloma Patients**
CAR-T therapy is typically recommended for multiple myeloma patients who have developed resistance to standard treatments (such as proteasome inhibitors, immunomodulatory drugs, and monoclonal antibodies) and are in their third line of treatment or beyond. In China, CAR-T therapy has broader indications and better efficacy.
### Suitable Patient Types:
-
Relapsed or refractory multiple myeloma patients, typically those who have undergone at least second-line treatments.
-
Patients with high-risk genetic characteristics, such as certain mutations (e.g., 17p deletion, t(4;14) translocation).
-
Patients unable to tolerate traditional treatments, including chemotherapy, other targeted therapies, stem cell transplantation, or immunomodulatory treatments, due to poor tolerance or severe side effects.
-
Patients who are BCMA-positive.
-
Patients whose disease continues to progress despite other treatments, especially chemotherapy.
-
Patients who are ineligible for stem cell transplantation.
-
Patients with severe symptoms not responsive to conventional treatments, such as bone pain, anemia, hypercalcemia, and kidney damage.
-
High tumor burden patients—experts generally reduce the tumor burden first before administering CAR-T therapy, often through bridging or sequential treatments.
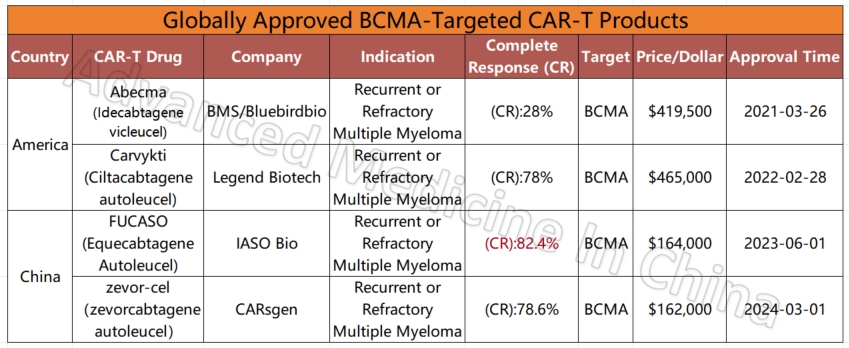
Among the four global CAR-T products targeting the BCMA marker in multiple myeloma, two Chinese products stand out for their efficacy and affordability. The most effective product currently is IASO Bio’s FUCASO (Equecabtagene Autoleucel), with a complete response (CR) rate of 82.4%.
## 2. **Patients in Good Physical Condition**
CAR-T therapy is essentially a powerful immunotherapy. Although it has remarkable efficacy, it also carries some risks of side effects, including cytokine release syndrome (CRS) and neurotoxic reactions. China’s CAR-T treatment system is highly developed, with established treatment plans and consensus. The country has extensive experience in managing CRS, neurotoxins, and side effects, keeping the risks very low. Moreover, the expert doctors at Advanced Medicine in China have substantial experience in managing these risks. Nonetheless, patients are typically required to have stable physical health to withstand the risks associated with the treatment.
– Overall good physical function; patients with heart, lung, liver, or kidney dysfunction may require a secondary evaluation by an expert team.
– Patients need to score 0-1 on the ECOG performance status scale, indicating that they are capable of daily activities and self-care.
## 3. **Patients with Financial and Time Support for CAR-T Therapy**
CAR-T therapy’s high cost and complex manufacturing process require patients and their families to have financial support. CAR-T therapy in China is significantly more affordable than in Western countries. While CAR-T treatments in the U.S. may cost $600,000 to $700,000, China’s approved CAR-T treatments cost around $100,000, about one-fifth to one-seventh of U.S. prices. Nevertheless, it remains a high-cost treatment, so patients need to be fully aware of the expenses involved.
China has invested heavily in CAR-T research in recent years. Many top hospitals and research institutions conduct related clinical trials. China accounts for over 50% of global CAR-T clinical trials. If a patient qualifies for a clinical trial, participating in it could be a more economical option, offering access to the latest CAR-T therapies, sometimes costing only tens of thousands of dollars or even free.
The CAR-T treatment process is relatively long. It involves collecting T cells, modifying them, re-injecting them, and close monitoring, requiring the patient to have enough time and patience to complete the entire process. The fastest known case took two weeks to complete all steps and discharge with complete response (CR), but generally, the process takes about four weeks or longer, depending on the patient’s condition.
For patients who can afford the treatment and have the time to complete it, CAR-T therapy in China is undoubtedly an attractive option.
## Conclusion
China’s leading position in CAR-T cell therapy technology and lower treatment costs make it a popular destination for multiple myeloma patients worldwide. It offers new hope for relapsed or refractory multiple myeloma patients. By working with experienced medical teams, patients can receive more personalized treatment plans and benefit from the rapid development of CAR-T therapy globally.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CancerTreatment #ChinaMedicalInnovation #Immunotherapy #MyelomaTreatment #AdvancedMedicine #RelapsedMyeloma #CancerResearch
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
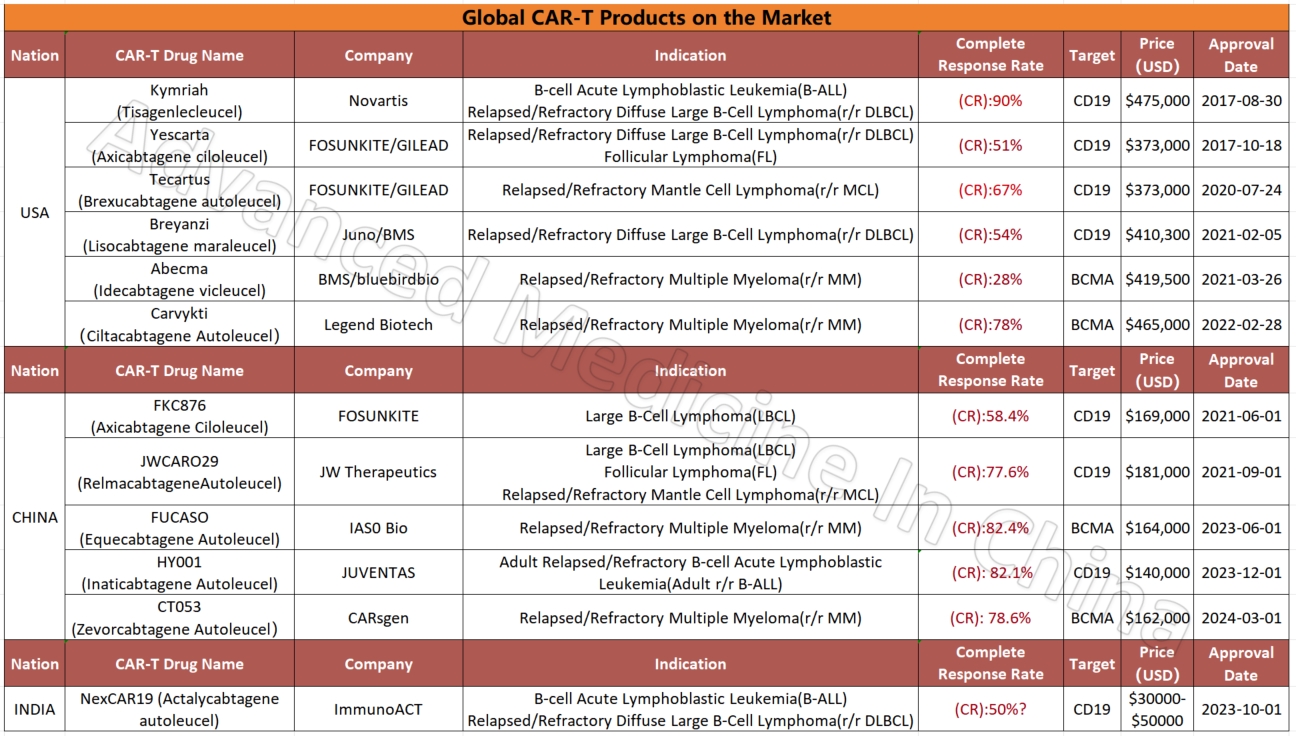
Global CAR-T Products on the Market and BCMA-Targeted CAR-T Products
Global CAR-T Products on the Market

CAR-T
Globally Approved BCMA-Targeted CAR-T Products
– Multiple Myeloma
Multiple Myeloma

 To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of Advanced Medicine in China for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

**Chinese Medical Team Releases Dual-Target CAR-T Data: 100% Complete Remission Rate in High-Risk MM Patients**
**Chinese Medical Team Releases Dual-Target CAR-T Data: 100% Complete Remission Rate in High-Risk MM Patients**

High-Risk MM
#CARTTherapy #MultipleMyeloma #BCMA #CD19 #JAMAOncology #MM #HRMM #DualTarget
Once again, a Chinese medical team is at the forefront of innovation, making significant strides in the field of CAR-T cell therapy. A recent study published in *JAMA Oncology* (B-Cell Maturation Antigen/CD19 Dual-Targeting Immunotherapy in Newly Diagnosed Multiple Myeloma) revealed remarkable results for the dual-target BCMA/CD19 CAR-T therapy in high-risk newly diagnosed multiple myeloma (NDMM) patients. In this study, all 19 participants achieved stringent complete remission (sCR) and were found to be minimal residual disease (MRD) negative, resulting in an astounding 100% remission rate.
**CAR-T Therapy: A Breakthrough in Hematologic Cancer Treatment**
Multiple myeloma (MM) is a challenging hematologic cancer, particularly for patients with high-risk features, where traditional treatments often show limited efficacy. CAR-T cell therapy is an innovative immunotherapy that reprograms a patient’s own T cells to recognize and attack cancer cells. In recent years, this therapy has shown significant success in treating leukemia, lymphoma, and relapsed/refractory MM (RRMM). The latest study further demonstrates that dual-target CAR-T therapy, focusing on BCMA and CD19, can significantly improve the prognosis for high-risk MM patients.
**The Impressive Results of China’s Dual-Target CAR-T Therapy**
The CAR-T therapy used in this study, GC012F, targets both B-cell maturation antigen (BCMA) and CD19 surface antigens, showing powerful anti-tumor effects. Every patient in the study not only achieved complete remission after treatment but also maintained a long-term MRD-negative status, meaning that traces of cancer cells were nearly undetectable post-therapy.
More importantly, the study highlighted the swift effectiveness of the Chinese GC012F therapy—patients reached their first complete remission in a median time of just 84 days, with MRD-negative status achieved in as little as 28 days. This rapid anti-tumor response provides a crucial treatment window for patients, significantly improving their prognosis.
**Safety and Future Prospects**
In addition to its impressive efficacy, GC012F demonstrated favorable safety. Only 27% of patients experienced mild to moderate cytokine release syndrome (CRS), and no cases of immune effector cell-associated neurotoxicity syndrome (ICANS) were observed. This is in stark contrast to previous studies in relapsed/refractory patients, where CRS rates were higher, highlighting the safety advantage of this therapy in newly diagnosed patients.
Though the study sample size was small, these findings bring new hope for high-risk multiple myeloma patients. As larger clinical trials are conducted and combination therapies are explored, this therapy has the potential to offer better survival outcomes for more patients.
**Conclusion**
China’s CAR-T cell therapy research continues to lead the way in hematologic cancer treatment. The success of the BCMA/CD19 dual-target CAR-T therapy not only demonstrates its strong efficacy in high-risk multiple myeloma but also provides a new direction for treating hematologic cancers globally. As research deepens, we anticipate that this groundbreaking therapy will bring new life-saving opportunities to more patients.
This breakthrough in Chinese CAR-T therapy represents not only scientific progress but also underscores China’s pivotal role in the global fight against cancer.
To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CancerTreatment #Immunotherapy #MedicalInnovation #CancerResearch #ChinaHealthcare #HematologicCancer #CancerBreakthrough
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Uncovering the Hidden Threat: How Serum Protein Electrophoresis Helps Diagnose Multiple Myeloma
**Uncovering the Hidden Threat: How Serum Protein Electrophoresis Helps Diagnose Multiple Myeloma**

Serum Protein Electrophoresis
As we age, it’s common to experience certain health issues—unexplained anemia, bone pain, fractures, nausea, or abnormal urine changes. Many may dismiss these symptoms as a result of aging, poor diet, or even mild illness. However, such seemingly unrelated signs could indicate a more serious condition: **Multiple Myeloma (MM)**.
**What is Multiple Myeloma (MM)?**
Multiple Myeloma is a type of blood cancer that originates in the bone marrow, where abnormal plasma cells multiply uncontrollably. These malignant cells produce large amounts of a protein called **M-protein** (monoclonal protein), leading to damage in various organs such as the kidneys and bones. The condition is complex, with different patients exhibiting diverse symptoms, often making it challenging to diagnose early. Key factors contributing to MM include genetic mutations and chromosomal changes, alongside abnormalities in the bone marrow microenvironment.
**Why is Early Detection Crucial?**
Over the past few decades, MM cases have steadily increased, particularly among older adults. Delayed diagnosis can result in severe complications, impacting the patient’s quality of life. Due to its subtle onset and wide range of symptoms, many MM patients may see multiple specialists without receiving the correct diagnosis. This makes raising awareness of MM screening critical—early detection can significantly improve survival and quality of life.
One of the most effective tools for early diagnosis is **Serum Protein Electrophoresis (SPE)**—a simple yet powerful lab test that can detect the presence of abnormal proteins, such as M-protein, in the blood.
**How Serum Protein Electrophoresis (SPE) Works**
SPE works by separating the proteins in your blood serum based on their size and electrical charge. Imagine it like children sliding down a playground slide, each going at different speeds depending on their size and shape. Similarly, proteins in the blood move at varying speeds in an electric field. This separation allows us to visualize distinct “protein bands” that represent different proteins like albumin and globulins. For MM patients, the presence of a sharp spike (M-protein) in the **gamma globulin region** serves as a telltale sign of the disease.
This method not only helps in identifying MM but also plays a key role in monitoring disease progression and assessing the effectiveness of treatment. By measuring the quantity of M-protein, doctors can gauge the severity of the disease and tailor therapies accordingly.
**Beyond Multiple Myeloma: Other Uses of SPE**
While SPE is essential for diagnosing MM, it is also a useful tool for identifying other conditions, including **liver diseases**, **kidney disorders**, and **chronic infections**. For example, in patients with liver cirrhosis, SPE often shows an increase in **beta and gamma globulin**, creating a distinctive pattern called a **beta-gamma bridge**. Similarly, kidney disease patients typically exhibit reduced albumin levels. By analyzing these patterns, doctors gain deeper insights into the underlying health issues.
**Other Diagnostic Tools for MM**
In addition to SPE, there are several other lab tests and imaging techniques that can help detect MM. These include **complete blood counts (CBC)**, **kidney function tests**, **calcium levels**, and imaging such as **X-rays** or **MRIs**. Research has shown that combining various tests, such as hemoglobin, globulin, HDL cholesterol, and uric acid, enhances the early detection of MM.
**A Complex Disease with Varied Outcomes**
Though multiple myeloma remains incurable, treatments can significantly extend survival, especially when caught early. If you or someone you know is experiencing unexplained symptoms like persistent anemia, unusual bone pain, or kidney issues, it’s essential to seek evaluation by a hematologist. Timely diagnosis and treatment are critical to improving both prognosis and quality of life.
**Stay informed, stay vigilant, and prioritize early screening** to protect yourself from the silent threat of multiple myeloma.
To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070 (Https://wa.me/+8613717959070)
Email: doctor.huang@globecancer.com
#MultipleMyeloma #BloodCancer #CancerAwareness #EarlyDiagnosis #SerumProteinElectrophoresis #HealthTips #MMResearch #CancerScreening #BloodTests #HealthyAging #MedicalBreakthrough
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Redefining Hope for High-Risk RRMM Patients Worldwide: A Singaporean Patient`s Journey to China for Groundbreaking CAR-T Therapy
Redefining Hope for High-Risk RRMM Patients Worldwide: A Singaporean Patient`s Journey to China for Groundbreaking CAR-T Therapy

Multiple Myeloma
#MultipleMyeloma #CAR_Therapy #CancerTreatment #HRMM #MM #RRMM #CART
In the fight against multiple myeloma (MM), the last few decades have seen significant advancements, yet the disease remains notoriously difficult to cure, particularly in patients with relapsed/refractory multiple myeloma (RRMM). These patients face enormous challenges, as their options become increasingly limited after multiple lines of therapy have failed. However, hope has emerged in the form of BCMA CAR-T therapy, offering deep remission and long-term survival for those who had nearly lost hope.
One such case is a 58-year-old woman from Singapore, who after exhausting all available treatments in her home country, found new hope in China`s innovative CAR-T therapy. Diagnosed with MM in May 2021 following a month of severe back pain, she underwent a series of treatments including CD38 monoclonal antibodies, immunomodulatory drugs (IMiDs), proteasome inhibitors (PI), and XPO-1 inhibitors. Unfortunately, these therapies failed to halt the progression of her disease, which had become highly resistant to treatment.
In December 2023, she traveled to Beijing Chaoyang Hospital, Capital Medical University, where Professor Chen Wenming took charge of her case. The patient was diagnosed with high-risk MM (IgG-κ type) and admitted to the hospital on November 25, 2023, for CAR-T therapy.
#### The Treatment Journey: A Detailed Overview
Given the patient’s refractory nature and multiple prior treatments, Professor Chen devised a tailored treatment plan to improve her chances of survival and quality of life. In November 2023, her lymphocytes were collected to prepare the CAR-T cells. During this period, she received two cycles of D-PACE (dexamethasone, cisplatin, doxorubicin, cyclophosphamide, and etoposide) chemotherapy in Singapore to control the extramedullary plasmacytoma.
In February 2024, she returned to Beijing for further evaluation, where her condition was assessed as showing minimal response (MR). She was then administered a lymphodepletion regimen of fludarabine and cyclophosphamide on February 29. The following month, she received a transfusion of BCMA CAR-T cells.
Within five days post-transfusion, the patient developed a fever, which peaked at 39°C. Fortunately, her oxygen saturation and heart rate remained normal, and she was diagnosed with Grade 1 cytokine release syndrome (CRS). After symptomatic treatment, her blood counts recovered by day 15, and she was discharged in stable condition.
Two weeks post-CAR-T therapy, her response was evaluated as a very good partial response (VGPR), and by the two-month mark, her condition had improved to complete remission (CR) with minimal residual disease (MRD) negativity.
#### Insights from Leading Experts
Professor Wee Joo Chng, a specialist in high-risk MM, noted the aggressive nature of the patient’s disease, marked by genetic abnormalities like 1q21+ and t(4;14). Despite the use of multiple potent therapies, including KRd, XVd, and Isa-Pd, the patient’s disease continued to progress rapidly. The emergence of the del(17p) mutation further complicated her prognosis, indicating the need for a novel therapeutic approach.
The FUMANBA-1 study has highlighted the effectiveness of China`s indigenous CAR-T product, Equecabtagene Autoleucel, in achieving deep remission and prolonging survival in RRMM patients. This case demonstrated the therapy`s potential to overcome poor prognostic factors and extend the patient’s survival. Notably, the patient experienced only mild CRS and no immune effector cell-associated neurotoxicity syndrome (ICANS) or infections during treatment. At the two-month follow-up, the patient’s condition had improved to CR with MRD negativity, suggesting that Equecabtagene Autoleucel could be a game-changer for high-risk RRMM patients.
#### A New Frontier in CAR-T Therapy
RRMM patients with double-hit characteristics often experience early relapse and progression, leading to shortened survival times. Traditional therapies, including IMiDs, PIs, and monoclonal antibodies, have failed to overcome these poor prognostic factors, indicating the urgent need for novel treatments. Real-world studies have shown that CAR-T therapy offers comparable progression-free survival (PFS) and overall survival (OS) rates in RRMM patients, regardless of high-risk cytogenetic abnormalities.
The FUMANBA-1 study revealed impressive outcomes for Equecabtagene Autoleucel in RRMM patients, with an overall response rate (ORR) of 98.9% and an MRD negativity rate of 97.8% among CAR-T-naive patients. The CR rate was 82.4%, and 81.7% of patients maintained MRD negativity for over a year.
Globally, four CAR-T products are currently available, and a recent study presented at the 2024 European Society for Blood and Marrow Transplantation (EBMT) compared the short- and long-term efficacy of these therapies. The study’s matching-adjusted indirect comparison (MAIC) analysis revealed that Equecabtagene Autoleucel had a 12-month PFS rate of 94.2%, higher than the 75% observed with Ciltacabtagene autoleucel (CARTITUDE-1 study). Furthermore, the 12-month sustained MRD negativity rate for Equecabtagene Autoleucel was 100%, compared to 53.1% for Ciltacabtagene autoleucel.
These findings suggest that Equecabtagene Autoleucel, a Chinese-developed BCMA CAR-T therapy, offers superior long-term efficacy compared to its U.S. counterpart. As the global community celebrates the first anniversary of its approval, Equecabtagene Autoleucel continues to bring hope to RRMM patients worldwide, further solidifying China’s leading role in the field of cellular therapy.

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp:+8613717959070
Email: doctor.huang@globecancer.com
#ChinaMedicalInnovation #GlobalHealth #MMResearch #PatientCare #InnovativeMedicine #CellTherapy #MedicalBreakthrough
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

A Miraculous Journey: Israeli Artist Finds Cure for Multiple Myeloma in Hangzhou China






The latest research findings of BCMA CAR-T therapy for multiple myeloma in 2023 | Equecabtagene Autoleucel (FUCASO)




Breakthrough medical research in China brings hope for multiple myeloma patients.
Eque-cel brings new treatment prospects for multiple myeloma.
From December 9th to 12th, the Annual American Society of Hematology Meeting (ASH 2023) was held in San Diego, USA. At this conference, the FUMANBA-1 study, led by Professors Chunrui Li from Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, and Lugui Qiu from the Institute of Hematology, Chinese Academy of Medical Sciences, conducted at 14 medical centers nationwide, demonstrated the efficacy and characteristics of sustained minimal residual disease (MRD) negativity in multiple myeloma (MM) patients treated with Eque-cel injection (CT103A). Their achievements were included in the oral presentation at the conference.

Current MM Treatment and Challenges of CAR-T Therapy CAR-T cell therapy holds significant importance in the treatment of multiple myeloma, overcoming patients’ immunodeficiency and tolerance issues. Currently, China has approved three CAR-T cell drugs targeting multiple myeloma. Compared to traditional treatments, CAR-T cell therapy enhances immune function without relying on the major histocompatibility complex (MHC), improving the activity of NK cells, T cells, and B cells while effectively activating T cells. However, despite significant advancements in CAR-T cell therapy in the MM field, some patients still struggle to maintain treatment effectiveness, leading to potential relapses, with antigen escape being one of the important mechanisms causing relapse.
Furthermore, the safety of CAR-T therapy is a major concern, including adverse reactions such as cytokine release syndrome (CRS), neurotoxicity, infections, B cell depletion, and hypogammaglobulinemia. Hence, monitoring patient indicators throughout the entire CAR-T treatment process, from pre-treatment to infusion, is crucial for timely intervention in adverse reactions.
100% MRD Negativity Rate for CR and Above Patients! Eque-cel Injection Brings Redemption for Multiple Myeloma
Eque-cel injection (CT103A) is a fully humanized CAR-T cell therapy drug targeting B cell maturation antigen (BCMA), approved by the National Medical Products Administration (NMPA) for adult refractory/relapsed multiple myeloma patients in China. In the Phase II FUMANBA-1 study, this treatment showed significant and sustained efficacy.
As of December 31, 2022, with a median follow-up time of 18.07 months, deep and sustained remissions were observed in 103 evaluable patients. The overall response rate (ORR) among these 103 patients was 96.1%, with a strict complete response (sCR)/complete response (CR) rate of 77.7%. Among 91 subjects with no previous CAR-T treatment history, the ORR was 98.9%, and the sCR/CR rate reached 82.4%, with a 12-month progression-free survival (PFS) rate of 85.5%.
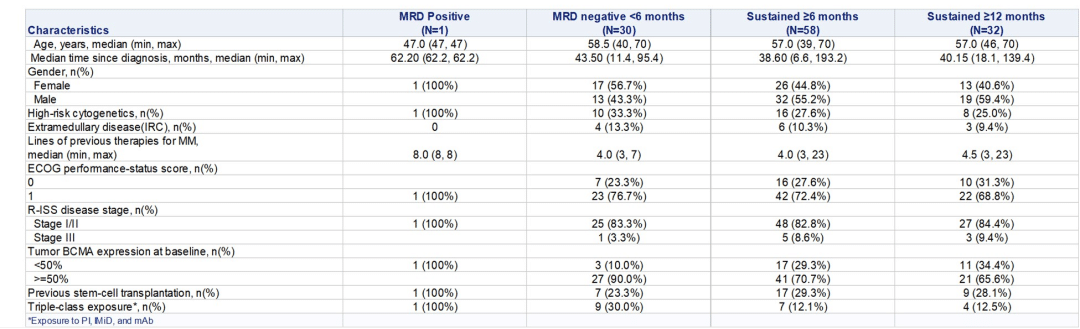
The minimal residual disease (MRD) negativity rate for the entire population was 94.2%, reaching 100% for CR and above patients. The median time to MRD negativity was 15 days, and 80.8% of patients maintained MRD negativity 12 months post-infusion. Moreover, the Eque-cel injection exhibited a relatively long median persistence in the body, lasting up to 307.5 days.
According to Professor Chunrui Li, “The sustained MRD-negative status is closely related to the improvement in PFS of patients receiving Eque-cel treatment.”
Professor Chunrui Li’s team studied the characteristics of patients maintaining MRD negativity for ≥6 months and ≥12 months in the FUMANBA-1 study. The research revealed that among 88 patients achieving MRD negativity, 78.4% maintained MRD negativity for at least 6 months, and 74.4% sustained MRD negativity for at least 12 months. Patients with sustained MRD negativity showed longer…

Professor Chunrui Li:
Chief Physician, Doctoral Supervisor
Secretary of the Hematology Department Party Committee, Deputy Director of Medicine Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology Professional
Focus: Immunotherapy for Hematological Malignancies
Member of the 11th Committee of the Plasma Cell Disease Professional Group of the Hematology Branch of the Chinese Medical Association
Chairman of the Expert Committee on Hematology (Hubei), Geriatrics Branch, Chinese Geriatrics and Gerontology Society
Standing Committee Member of the Geriatric Medicine Branch, Chinese Geriatrics and Gerontology Society
Youth Committee Member of the Oncology Branch, Chinese Anti-Cancer Association Committee Member of the Myeloma and Plasma Cell Disease Professional Group, 5th Committee of the Chinese Society of Clinical Oncology (CSCO) Leukemia Alliance & Lymphoma Alliance
Vice Chairman of the Blood Branch of the Hubei Medical Immunology Association Principal Investigator of four National Natural Science Foundation projects; published more than 20 SCI papers as first author or corresponding author, including Blood.
#EqueCelTreatment #MMResearch #CARTTherapy #MRDNegativity #CancerTreatment #MedicalBreakthrough #FUMANBA1Study #Immunotherapy #CancerResearchUpdate #CancerResearch #ASH2023 #BloodCancer #EqueCel #MRD #MultipleMyeloma
