Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

The significance of the 11 CD19 and BCMA CAR-T therapies currently approvedin China and the U.S. forpatients with hematologic malignancies.
The significance of the 11 CD19 and BCMA CAR-T therapies currently approvedin China and the U.S. forpatients with hematologic malignancies.
目前中美上市的11款CD19和BCMA CAR-T对血液瘤患者的意义
Expert:
**Jing Pan**
**Associate Chief Physician**
**Department of Pediatric Hematology, Beijing Gaobo Hospital**
**Professional Memberships:**
– Member, Pediatric Oncology Professional Committee of the Chinese Anti-Cancer Association
– Youth Committee Member, Hematologic Oncology Professional Committee of the Chinese Anti-Cancer Association
– Youth Committee Member, Clinical Application Professional Committee of the Chinese Medical Biotechnology Association
**Expertise:**
Dr. Pan currently manages an 80-bed pediatric hematology unit and has extensive experience in pediatric hematology, particularly in CAR-T cell immunotherapy, with nearly 10 years of experience in the field. She is dedicated to stratified CAR-T treatment, optimizing the management of complications during CAR-T therapy, and establishing an efficacy monitoring system post-treatment. Dr. Pan and her team have accumulated one of the largest single-center case collections globally, particularly in the areas of sequential CAR-T therapy for improving long-term outcomes in B-ALL and in exploring autologous and allogeneic CD5, CD7 CAR-T therapy for T-ALL/LBL.
Her related clinical research on CD7 CAR-T, CD19 CAR-T, CD22 CAR-T, and CD19-22 sequential CAR-T has been published in leading international journals such as *Lancet Oncology*, *JCO*, *Blood*, *JHO*, and *Leukemia*. Additionally, she has frequently presented the latest advancements in her team’s immunotherapy research at both domestic and international conferences, including ASCO, ASH, EHA, and JSH.

 To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#cart #carttherapy #CAR_T #leukemia #lymphoma #cancer #tumor #bloodcancer
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
BCMA CAR-T Therapy Paves New Path for Treating High-Risk Multiple Myeloma
**BCMA CAR-T Therapy Paves New Path for Treating High-Risk Multiple Myeloma**

Multiple Myeloma
Multiple Myeloma (MM) is a malignant tumor originating from plasma cells, known for its complex biology and treatment resistance, presenting significant challenges to patients’ survival. For high-risk MM, especially in cases of relapse and refractory disease after multiple lines of therapy, treatment options become critically important. Recently, a team led by Professor Qian from a Chinese Hospital successfully treated a high-risk relapsed/refractory MM (RRMM) patient using BCMA CAR-T cell therapy, offering new hope for difficult-to-treat cases.
**Patient Overview**
The patient, a 56-year-old woman, initially sought treatment for persistent anemia and back pain. Baseline examinations revealed significant abnormalities, including a hemoglobin level of 67g/L, thrombocytopenia, and abnormal bone marrow findings. She was diagnosed with IgG-kappa type MM at stage ISS III/R-ISS III, with cytogenetic abnormalities and high-risk factors such as t(14;16) and 1q21 amplification. The disease rapidly progressed despite multiple lines of therapy, including VRd and DVD regimens, with external manifestations of extramedullary disease (EMD), indicating a poor prognosis.
**Treatment Journey**
The patient initially underwent VRd induction therapy, followed by DVD treatment when the disease progressed. Despite efforts, she developed multiple extramedullary lesions, leading to the use of the Dara+DECP regimen and autologous stem cell transplantation (ASCT). While these treatments provided partial remission, the disease relapsed within months, with extramedullary involvement further complicating the prognosis.
Given the poor prognosis and lack of effective treatments for EMD in MM, the decision was made to pursue BCMA CAR-T cell therapy, specifically with **Equecabtagene Autoleucel**, China’s first CAR-T product for treating MM. This therapy has demonstrated an impressive overall response rate (ORR) of 100% in patients with extramedullary disease, with a complete remission (CR) rate of 78.6%.
**CAR-T Therapy and Outcomes**
Following preconditioning with FC regimen, the patient underwent BCMA CAR-T therapy. After CAR-T infusion, the patient experienced mild cytokine release syndrome (CRS), which was successfully managed with supportive care. Over the course of three months, significant clinical improvements were observed. PET-CT scans showed no residual disease, and bone marrow biopsies were negative for clonal plasma cells. The patient achieved stringent complete remission (sCR).
Eight months after CAR-T therapy, follow-up results continue to show no evidence of disease, with the patient maintaining CR. This case highlights the long-lasting anti-tumor effects of Equecabtagene Autoleucel, a fully human BCMA CAR-T product, which offers low immunogenicity and sustained CAR-T cell persistence in vivo.
**Implications for Future Treatment**
This success story offers new hope for patients with high-risk and refractory MM, especially those with extramedullary involvement. It also provides valuable clinical insights into the use of BCMA CAR-T therapy as a promising treatment strategy. The case demonstrates that for patients with high-risk MM, comprehensive risk assessment considering genetic characteristics, treatment responses, and future treatment plans is essential for personalized care.
In conclusion, the application of Equecabtagene Autoleucel CAR-T therapy represents a significant breakthrough in the treatment of high-risk, refractory MM, particularly in cases involving extramedullary disease. This innovative approach not only extends survival but also enhances the quality of life for patients who have exhausted other treatment options, marking a new chapter in the fight against multiple myeloma.
🎉🎉To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#MultipleMyeloma #CAR_Therapy #BCMA #CancerTreatment #InnovativeMedicine #Immunotherapy #ExtramedullaryDisease #RelapsedMM #ChinaHealthcare #GlobalHealth
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Uncovering the Hidden Threat: How Serum Protein Electrophoresis Helps Diagnose Multiple Myeloma
**Uncovering the Hidden Threat: How Serum Protein Electrophoresis Helps Diagnose Multiple Myeloma**

Serum Protein Electrophoresis
As we age, it’s common to experience certain health issues—unexplained anemia, bone pain, fractures, nausea, or abnormal urine changes. Many may dismiss these symptoms as a result of aging, poor diet, or even mild illness. However, such seemingly unrelated signs could indicate a more serious condition: **Multiple Myeloma (MM)**.
**What is Multiple Myeloma (MM)?**
Multiple Myeloma is a type of blood cancer that originates in the bone marrow, where abnormal plasma cells multiply uncontrollably. These malignant cells produce large amounts of a protein called **M-protein** (monoclonal protein), leading to damage in various organs such as the kidneys and bones. The condition is complex, with different patients exhibiting diverse symptoms, often making it challenging to diagnose early. Key factors contributing to MM include genetic mutations and chromosomal changes, alongside abnormalities in the bone marrow microenvironment.
**Why is Early Detection Crucial?**
Over the past few decades, MM cases have steadily increased, particularly among older adults. Delayed diagnosis can result in severe complications, impacting the patient’s quality of life. Due to its subtle onset and wide range of symptoms, many MM patients may see multiple specialists without receiving the correct diagnosis. This makes raising awareness of MM screening critical—early detection can significantly improve survival and quality of life.
One of the most effective tools for early diagnosis is **Serum Protein Electrophoresis (SPE)**—a simple yet powerful lab test that can detect the presence of abnormal proteins, such as M-protein, in the blood.
**How Serum Protein Electrophoresis (SPE) Works**
SPE works by separating the proteins in your blood serum based on their size and electrical charge. Imagine it like children sliding down a playground slide, each going at different speeds depending on their size and shape. Similarly, proteins in the blood move at varying speeds in an electric field. This separation allows us to visualize distinct “protein bands” that represent different proteins like albumin and globulins. For MM patients, the presence of a sharp spike (M-protein) in the **gamma globulin region** serves as a telltale sign of the disease.
This method not only helps in identifying MM but also plays a key role in monitoring disease progression and assessing the effectiveness of treatment. By measuring the quantity of M-protein, doctors can gauge the severity of the disease and tailor therapies accordingly.
**Beyond Multiple Myeloma: Other Uses of SPE**
While SPE is essential for diagnosing MM, it is also a useful tool for identifying other conditions, including **liver diseases**, **kidney disorders**, and **chronic infections**. For example, in patients with liver cirrhosis, SPE often shows an increase in **beta and gamma globulin**, creating a distinctive pattern called a **beta-gamma bridge**. Similarly, kidney disease patients typically exhibit reduced albumin levels. By analyzing these patterns, doctors gain deeper insights into the underlying health issues.
**Other Diagnostic Tools for MM**
In addition to SPE, there are several other lab tests and imaging techniques that can help detect MM. These include **complete blood counts (CBC)**, **kidney function tests**, **calcium levels**, and imaging such as **X-rays** or **MRIs**. Research has shown that combining various tests, such as hemoglobin, globulin, HDL cholesterol, and uric acid, enhances the early detection of MM.
**A Complex Disease with Varied Outcomes**
Though multiple myeloma remains incurable, treatments can significantly extend survival, especially when caught early. If you or someone you know is experiencing unexplained symptoms like persistent anemia, unusual bone pain, or kidney issues, it’s essential to seek evaluation by a hematologist. Timely diagnosis and treatment are critical to improving both prognosis and quality of life.
**Stay informed, stay vigilant, and prioritize early screening** to protect yourself from the silent threat of multiple myeloma.
To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070 (Https://wa.me/+8613717959070)
Email: doctor.huang@globecancer.com
#MultipleMyeloma #BloodCancer #CancerAwareness #EarlyDiagnosis #SerumProteinElectrophoresis #HealthTips #MMResearch #CancerScreening #BloodTests #HealthyAging #MedicalBreakthrough
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Hematologic Tumors – Detection: Unveiling the Mysteries of the Disease
** Hematologic Tumors
Hematologic Tumors ** –
** –  Detection
Detection :
:
Unveiling the Mysteries of the Disease
Hematologic tumors are a group of malignant diseases caused by the abnormal proliferation of blood-forming cells, affecting the bone marrow, blood, and various organs and tissues throughout the body. Common types of hematologic tumors include leukemia, myelodysplastic syndromes, lymphoma, multiple myeloma, and myeloproliferative neoplasms.
### Causes of Hematologic Tumors
The development of hematologic tumors is highly complex. Key factors include genetic mutations, immune system abnormalities, radiation exposure, contact with harmful chemicals, viral infections, and hereditary factors. Additionally, unhealthy lifestyle habits, prolonged psychological stress, and poor environmental conditions can increase the risk of these diseases.
In recent years, due to global population aging and modern lifestyle changes, the incidence of hematologic tumors has been on the rise. In China, leukemia and lymphoma rank among the top malignant tumors in terms of incidence and mortality. Therefore, early detection and accurate diagnosis of hematologic tumors are crucial for improving patient outcomes.
### Detection Methods for Hematologic Tumors
Since early symptoms of hematologic tumors are often subtle, many patients are diagnosed in the late stages of the disease. Thus, early detection is essential. The medical field has developed a variety of advanced and precise diagnostic methods to detect and confirm hematologic tumors.
#### 1. **Blood Tests**
A complete blood count (CBC) is a fundamental tool for detecting hematologic tumors. By analyzing the quantity and morphology of red blood cells, white blood cells, and platelets, physicians can preliminarily identify abnormalities. For example, leukemia patients often exhibit abnormal increases or decreases in white blood cells, along with immature cells in the bloodstream.
#### 2. **Bone Marrow Biopsy**
Bone marrow biopsy is a key diagnostic method for hematologic tumors. By analyzing the hematopoietic cells from a bone marrow sample, physicians can determine the presence of abnormal or malignant cell proliferation. This procedure not only confirms the type of hematologic tumor but also helps assess disease progression and treatment efficacy.
#### 3. **Flow Cytometry**
Flow cytometry is an efficient method for detecting hematologic tumors. It rapidly analyzes characteristic markers on the surface of cells, helping doctors identify and quantify abnormal cells. It plays an essential role in diagnosing diseases such as leukemia and lymphoma.
#### 4. **Genetic Testing**
Genetic mutations are a critical factor in many hematologic tumors. Genetic testing can reveal the mutation profile within a patient’s cells, enabling doctors to better understand the underlying cause of the disease and formulate personalized treatment plans. With advancements in molecular biology, genetic testing has become a cornerstone of precision medicine.
#### 5. **Imaging Tests**
For certain aggressive hematologic tumors, imaging tests like CT, PET-CT, and MRI help doctors assess the extent and spread of the disease. These techniques are particularly valuable in staging and evaluating the treatment response of lymphomas.
### Conclusion
The detection of hematologic tumors has become increasingly accurate, greatly improving early diagnosis rates and treatment outcomes. By effectively utilizing blood tests, bone marrow biopsies, flow cytometry, genetic testing, and imaging, patients can receive earlier diagnoses and more tailored treatment plans. With continuous advances in medical technology, the cure rates for hematologic tumors are expected to rise, bringing hope for better health to more patients.

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp:+8613717959070
(Https://wa.me/+8613717959070)
Email: doctor.huang@globecancer.com
#HematologicCancer #BloodCancer #CancerDetection #LeukemiaAwareness #LymphomaAwareness #MultipleMyeloma #CancerDiagnosis #GeneticTesting #EarlyDetection #FlowCytometry #BoneMarrowBiopsy #CancerResearch #PrecisionMedicine #CancerTreatment #Immunotherapy #Oncology #CancerAwareness #HealthInnovation #MedicalAdvancements #CancerCare
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

**Treatment for Multiple Myeloma: China’s BCMA×CD3 Bispecific Antibody Proposed for Breakthrough Therapy Designation**
**Treatment for Multiple Myeloma: China’s BCMA×CD3 Bispecific Antibody Proposed for Breakthrough Therapy Designation**

Multiple Myeloma
#MultipleMyeloma #BCMAxCD3 #BispecificAntibody #Immunotherapy #GR1803 #NMPA #RRMM
Recently, GR1803, an injectable solution developed by Genrix Bio, has been proposed for the “Breakthrough Therapy Designation” by the Center for Drug Evaluation (CDE) under China’s National Medical Products Administration (NMPA). This innovative drug targets relapsed and refractory multiple myeloma (RRMM), especially for patients who have undergone at least three lines of treatment, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody.
GR1803 is a novel bispecific antibody that simultaneously binds to BCMA and CD3 antigens. Its unique design allows for the effective activation of T-cells to attack tumor cells while minimizing non-specific T-cell activation, reducing potential side effects. In its Phase I clinical trial for RRMM, GR1803 demonstrated an overall objective response rate (ORR) of 85%, with an ORR of 100% in patients with extramedullary plasmacytoma (EMM).
The clinical performance of this product is remarkable, as evidenced by data presented at the 2024 European Hematology Association (EHA) annual meeting. As of January 2024, 40 trial participants have been enrolled, with an ORR of 85%. Notably, in the 180 ug/kg dose group, the median follow-up duration was 15 weeks, and 96% of the patients achieved a significant response.
What’s particularly noteworthy is the rapid response time; patients showed signs of improvement within a median of just 3 weeks. This suggests that GR1803 not only provides effective disease control in the short term but also continues to enhance patients’ conditions as treatment progresses.
The results from Genrix Bio’s research highlight that GR1803 offers new hope for RRMM patients, particularly for those who are unresponsive to conventional therapies. Its high response rate and favorable safety profile suggest that this drug could play a critical role in future cancer treatments.
The development of such groundbreaking drugs and the advancement of breakthrough therapies reflect China’s growing innovation in the global fight against cancer, offering new treatment options for patients worldwide.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#MultipleMyeloma #CancerTreatment #BreakthroughTherapy #Immunotherapy #BiotechInnovation #GenrixBio #BCMAxCD3 #CancerResearch #OncologyNews #PharmaInnovation
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China’s first BCMA CAR-T therapy successfully treats an overseas patient with advanced relapsed multiple myeloma
China’s first BCMA CAR-T therapy successfully treats an overseas patient with advanced relapsed myeloma.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp:+8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#MultipleMyeloma #CAR-T #CancerTreatment #MedicalBreakthrough #Oncology #HealthcareInnovation #BCMACART
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China’s Fully Human BCMA CAR-T Therapy: Bringing New Hope for High-Risk Relapsed/Refractory Multiple Myeloma
China’s Fully Human BCMA CAR-T Therapy: Bringing New Hope for High-Risk Relapsed/Refractory Multiple Myeloma

Mutiple Myeloma
Multiple Myeloma (MM) is a complex and aggressive cancer originating from plasma cells, posing significant treatment challenges due to its resistance to therapy. For patients with high-risk MM, especially those who experience relapse after multiple lines of treatment, finding an effective therapy is critical. Recently, Chinese medical team successfully treated a case of high-risk relapsed/refractory MM (RRMM) using fully human BCMA CAR-T cell therapy, offering new hope for such difficult cases.
**Case Overview**
The patient, a 56-year-old woman, presented with anemia during a routine check-up and was later diagnosed with MM. Despite undergoing several treatment regimens, including VRd (bortezomib, lenalidomide, dexamethasone) and Dara-DECP followed by autologous stem cell transplantation (ASCT), the disease continued to progress, with extramedullary disease (EMD) manifestations complicating the case.
**Challenges and Treatment Journey**
After initial treatments failed to achieve long-term remission, the patient’s condition worsened, with new lesions detected in the pancreas and multiple subcutaneous nodules indicating possible metastasis. Given the aggressive nature of the disease and the presence of EMD, which is associated with a poor prognosis, the medical team opted for a BCMA-targeted CAR-T cell therapy using Iquarense (Ikaros CAR-T), the first CAR-T product approved for MM treatment in China.
**CAR-T Therapy and Results**
Following pre-conditioning with a fludarabine-cyclophosphamide (FC) regimen, the patient received the BCMA CAR-T therapy. The treatment was well-tolerated, with only mild cytokine release syndrome (CRS) observed. Remarkably, within three months post-treatment, PET-CT scans showed no signs of active disease, and the patient achieved a complete response (CR), which has been sustained for eight months.
**Significance and Future Implications**
This case highlights the potential of BCMA CAR-T therapy as a powerful option for patients with high-risk, relapsed/refractory MM, particularly those with EMD. The successful outcome not only provides new hope for patients facing similar challenges but also contributes valuable insights for future treatment strategies. Iquarense, with its low immunogenicity and prolonged persistence in the body, represents a promising advance in the fight against this formidable disease.
For patients with high-risk MM, it is crucial to consider genetic factors, treatment response, and overall disease dynamics when selecting a therapeutic approach. As this case demonstrates, BCMA CAR-T therapy offers a viable path forward, particularly for those with limited options due to disease progression or EMD.
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
(Http://wa.me/+8613717959070)
Email: doctor.huang@globecancer.com
#BloodCancerTreatment #CAR_TCellTherapy #CancerBreakthrough #Immunotherapy #BCMACART #MedicalAdvancements #CancerSurvivorship #ChinaMedicalInnovation #HopeForCancerPatients
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Multiple Myeloma Solution ** Breakthroughs and Hope in Blood Cancer Treatment**
Multiple Myeloma Solution
** Breakthroughs and Hope in Blood Cancer Treatment**

Mutiple Myeloma
Multiple myeloma is a malignant blood cancer caused by the abnormal proliferation of plasma cells in the bone marrow, often affecting the bones, kidneys, and various organs and systems. With the advancement of modern medical technology, the understanding and treatment of multiple myeloma have greatly improved, bringing unprecedented hope to patients.
### Causes and Risk Factors of Multiple Myeloma
The causes of multiple myeloma are complex, typically involving multiple factors such as genetic mutations, immune system abnormalities, exposure to harmful chemicals, viral infections, and genetic predisposition. Additionally, lifestyle and environmental factors like smoking, obesity, and certain occupational exposures are also believed to increase the risk of developing the disease. With an aging population, the incidence of multiple myeloma is on the rise.
### Evolution of Treatment: Traditional and Innovative Approaches
Although multiple myeloma was once considered a difficult-to-treat disease, recent advancements in treatment methods have made significant progress. Traditional treatments include chemotherapy, radiation therapy, and bone marrow transplantation, which have extended patients’ survival to some extent but come with limited efficacy and significant side effects.
However, with advances in medical technology, new treatment options have emerged. Monoclonal antibodies have played a key role in treating multiple myeloma, targeting cancer cells precisely while reducing harm to healthy cells. Antibody-drug conjugates take this a step further by delivering chemotherapy drugs directly to cancer cells, enhancing efficacy while minimizing side effects.
Small molecule targeted drugs represent another breakthrough. These drugs inhibit specific genes or proteins in cancer cells, preventing their growth and spread. For example, BCL-2 inhibitors and protein kinase inhibitors have shown promising results in clinical trials, offering more treatment options.
### Immunotherapy: Leading the Future of Hope
Immunotherapy is becoming increasingly important in the treatment of blood cancers, especially in the field of multiple myeloma. By activating the patient’s own immune system to attack cancer cells, immunotherapy holds great promise. Among the most forward-looking therapies is CAR-T cell therapy. This treatment involves genetically modifying a patient’s T cells to recognize and kill cancer cells, and it has achieved remarkable results in multiple myeloma patients.
In recent years, China has made significant progress in the research and application of immunotherapy. Particularly in the field of blood cancers, China has developed a comprehensive treatment system with established protocols and consensus. The BCMA-targeted CAR-T cell therapy has shown deep and lasting efficacy in treating multiple myeloma, greatly improving patients’ disease-free survival rates. For instance, China’s fully human BCMA-targeted CAR-T product, Equecabtagene Autoleucel, has demonstrated the best data among CAR-T products for multiple myeloma, with a complete remission rate of 82.4%.
### Looking Ahead
With the continuous emergence of new drugs and therapies, the treatment of multiple myeloma is moving towards individualized and precision medicine. Patients not only experience extended lifespans but also regain their quality of life, returning to normalcy in their everyday lives and work. As medical advancements continue, there is reason to believe that multiple myeloma will no longer be an incurable disease, and every patient will be able to embrace a brighter future.
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070 (Http://wa.me/+8613717959070)
Email: doctor.huang@globecancer.com
#BloodCancerTreatment #CAR_TCellTherapy #CancerBreakthrough #Immunotherapy #BCMACART #MedicalAdvancements #CancerSurvivorship #ChinaMedicalInnovation #HopeForCancerPatients
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
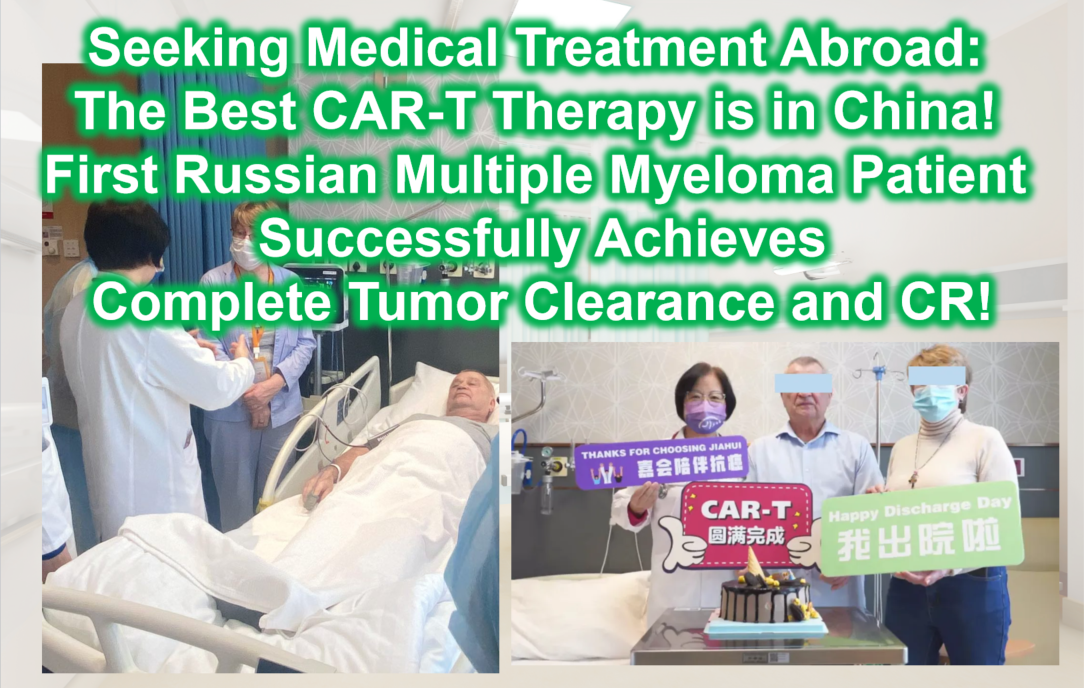
Seeking Medical Treatment Abroad: The Best CAR-T Therapy is in China! The First Russian Multiple Myeloma Patient Successfully Achieves Complete Tumor Clearance and CR!
**Seeking Medical Treatment Abroad: The Best CAR-T Therapy is in China! The First Russian Multiple Myeloma Patient Successfully Achieves Complete Tumor Clearance and CR!**

patient story
Over the five years following his diagnosis of high-risk multiple myeloma, Mr. F endured relentless pain. Despite undergoing various chemotherapy regimens and two transplants, he could never completely escape the nightmare of unpredictable tumor recurrence and progression. Fortunately, by the end of 2023, his condition temporarily stabilized. Accompanied by top hematology experts from Russia, Mr. F traveled to China to receive CAR-T therapy, achieving optimal results with complete tumor eradication and CR (complete remission).
Achieved CR! In February 2024, Mr. F and his family successfully returned to Russia to resume their happy lives.
(Note: To protect patient privacy, pseudonyms are used in the text, and the medical details and treatment process have been slightly modified.)

**Common Back Pain Turned Out to Be High-Risk Multiple Myeloma**
Mr. F, 63, is the vice principal of a technical college in St. Petersburg, Russia. He has a successful career and a happy family with his wife, one son, and two daughters. In the months leading up to Christmas 2017, Mr. F experienced worsening back pain, with severe pain in the lumbar region. Accompanied by his family, he went to the hospital for a thorough examination. The final diagnosis was multiple myeloma, with osteolytic lesions in more than three areas. The doctor informed Mr. F that, based on the comprehensive assessment of various test results, his myeloma was classified as high-risk (IgG-type, DS-Stage IIIA, ISS-Stage II), making it difficult to treat, prone to relapse, and with a poor prognosis. This devastating news struck suddenly, plunging the entire family into shock and panic. In an interview, Mr. F said, “In Chinese culture, Confucius said, ‘When Heaven is about to place a great responsibility on a man, it always first frustrates his spirit,’ viewing it as a life test. In Western culture, it is seen as God’s punishment, a trial one must endure, with the outcome entirely dependent on how one faces these challenges. I must choose to fight!”
**Relapse and Progression: The Unshakeable Nightmare for Multiple Myeloma Patients**
After two days of shock and panic, Mr. F decided to pull himself together and seek the best treatment to fight the tumor. Mr. F and his family consulted many doctors and clinics in Spain, Munich, and Heidelberg. Upon learning that CAR-T therapy had remarkable curative effects on hematologic malignancies, they felt a glimmer of hope, which was soon extinguished. At that time, very few countries offered CAR-T therapy, mostly in developed nations, and the exorbitant cost of nearly a million dollars made it unaffordable even for the well-off Mr. F. To control the disease as quickly as possible, Mr. F underwent traditional CVD therapy in Russia at the beginning of 2018, followed by his first autologous bone marrow transplant, achieving partial remission (PR). Unfortunately, just ten months later, his multiple myeloma relapsed. After switching to a second-line chemotherapy regimen, he underwent a second autologous bone marrow transplant. Despite receiving the most aggressive treatment and enduring life-threatening side effects, tumor lesions persisted in his body, like a ticking time bomb that could explode at any moment. Although maintenance therapy temporarily stabilized the disease, Mr. F and his family remained vigilant. They knew that high-risk myeloma was likely to relapse and progress again in the short term. If the disease worsened once more, they would face a desperate situation with no available treatments.

**Global Comparison Shows the Best CAR-T Therapy is in China!**
To completely rid himself of cancer and escape the nightmare of constant relapse and deterioration, five years later, Mr. F and his family once again sought the possibility of a cure through CAR-T therapy worldwide. They conducted extensive research on the CAR-T therapies already on the market and consulted numerous hospitals and experts in Europe, including in India and Israel. To his surprise, despite five years passing, there had been no significant progress in these countries. Almost every expert mentioned that Chinese hospitals had made tremendous advancements in the CAR-T field, surpassing even those in Europe and the United States. Especially for multiple myeloma, on June 30, 2023, China introduced the world’s first fully human BCMA CAR-T therapy, Iquilence. Mr. F and his attending physician immediately contacted Shanghai Jiahui International Hospital in China for an initial remote consultation. Jiahui Hospital, an internationally aligned oncology center with a professional multidisciplinary MDT team, conducted a preliminary evaluation. They concluded that the Iquilence (BCMA CAR-T) treatment plan would be most beneficial and developed a comprehensive plan, addressing all questions and concerns of Mr. F and the Russian experts, ultimately reaching a consensus on the treatment plan.
Professor Li Hua, Director of Oncology at Jiahui International Cancer Center, and his team of Russian hematology experts discussed Mr. F’s treatment plan. Although Mr. F was somewhat aware of China’s medical advancements, he couldn’t imagine that China’s healthcare had surpassed that of the developed Western countries. Therefore, in November 2023, Mr. F’s attending physician, a renowned Russian hematology expert, accompanied him to China to evaluate the feasibility of Chinese CAR-T therapy on-site. Their first stop upon arriving in China was to meet with Jiahui Hospital’s international multidisciplinary treatment team and tour the medical environment that adheres to international nursing standards.

Professor Li Hua, Director of Jiahui International Cancer Center, and his team provided a detailed introduction to the CAR-T treatment plan. Compared to traditional treatments, CAR-T has unique advantages. Firstly, it is a highly personalized treatment that adjusts the patient’s immune system through genetic engineering, providing a more precise response to cancer treatment. Secondly, CAR-T shows significantly higher efficacy in complex cases, which traditional treatments cannot achieve. Iquilence has an overall response rate of nearly 99% and a complete response rate of up to 82.4%. More importantly, fully human CAR-T not only maintains the patient’s quality of life but also provides more durable treatment effects. Additionally, a one-time infusion treatment achieves rapid and effective results, reducing the pain and burden during the treatment process and bringing hope for a cure.+

The second stop for Mr. F and the Russian experts was a visit to the production facility of the world’s first fully human BCMA CAR-T therapy, Iquilence. Mr. F and the Russian experts were impressed to learn that this exceptional therapy was not only entirely developed in-house, but the lentiviral vector was also independently developed. The facility boasts a complete end-to-end development platform and a self-built manufacturing base.

Finally, Mr. F overcame all his concerns and was convinced that China’s fully human BCMA CAR-T solution was world-leading. He decided to undergo treatment in China.
All Tumors Cleared for the First Time, Advanced Myeloma Achieves CR!
In December 2023, just before another Christmas five years after his diagnosis, Mr. F and his family arrived in China, full of hope for a cure, to officially begin his CAR-T journey. At Shanghai Jiahui International Hospital, Mr. F underwent thorough examinations, and after meeting all the requirements, he successfully underwent apheresis, the process of collecting T cells. These cells were then transported back to the factory within 24 hours to create a precisely targeted anti-cancer weapon tailored for Mr. F.
On January 20, 2024, he received lymphodepleting chemotherapy with fludarabine and cyclophosphamide.
On January 25, 2024, the CAR-T cells, carrying the hopes of Mr. F’s entire family, were transported to him by the pharmacy team using professional cold chain logistics. After multiple inspections, unpacking, and reactivation, a small bag of milky white liquid was infused back into Mr. F. The doctors explained that this liquid contained hundreds of billions of “special forces” T cells, which, once in the body, would begin an intense sweep to eradicate the myeloma cells completely.
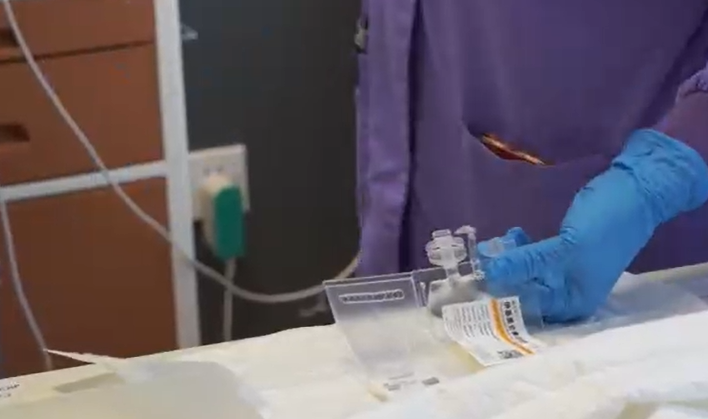
The infusion process went smoothly. What deeply moved Mr. F and his family was that whenever he felt anxious, the medical team would hold his hand, giving him confidence and strength.
Although a mild cytokine release syndrome (CRS), which is common after CAR-T therapy, occurred post-infusion, the medical team at Jiahui Hospital was very experienced in handling this. With symptomatic treatment, the symptoms improved in about a week. On the 14th day after the infusion, the evaluation showed that the CAR-T cells had expanded effectively in Mr. F’s body, reaching 10^9.
Mr. F said that, unlike the two painful transplant experiences, despite knowing that CAR-T therapy has side effects, the medical team clearly informed him about the reactions he might have at different stages and assured him that the medical staff would address them immediately. As a result, he felt no concerns or fears.
Before the Chinese New Year in 2024, Mr. F was discharged and returned to Russia for further follow-up in his home country. Professor Li Hua said, “The treatment results met our expectations, with significant improvements in key indicators such as serum M protein, free light chains, and disease symptoms. All functional statuses have improved.”
In May 2024, the PET-CT results showed that Mr. F’s lesions had disappeared, and he finally achieved complete remission (CR). This means that after battling multiple myeloma for six years, he has finally won!
A Brighter Future: China Will Become the Best Treatment Destination for Advanced Myeloma Patients!
Now, Mr. F not only resumes his happy and fulfilling life, but he and his wife have also returned to their beloved careers. He currently serves as the General Manager of the UNESCO Mineral Resources Education Center.
Mr. F said, “When I came to China and saw the medical conditions and experienced the best care firsthand, I felt a deep sense of pride for China. It is regrettable that Russia is currently unable to achieve this level of care. However, I will bring my experiences here back to Russia and share them. China’s medical system is world-leading, and choosing to come to China was the right decision!”

In an interview, Mr. F’s wife said, “Although we are past retirement age, we both have very important careers. This CAR-T treatment in China has given us a very precious experience. We have more time to listen to each other’s thoughts, support each other, and get through difficult times together.”

Professor Li Hua, Director of the International Oncology Hospital and Director of the Department of Oncology, said in an interview:
“Clinical research on CAR-T therapy started relatively early in China. Especially since 2020, the number of CAR-T clinical trials in China has far surpassed that in the United States, giving us extensive experience from laboratory research to clinical application.
Moreover, CAR-T treatment in China is more cost-effective, providing patients with a more competitive option.
Most importantly, China has sufficient commercial-quality production capacity. Patients can receive treatment quickly without waiting, which is crucial for those with malignant hematologic diseases who need timely treatment.”
Professor Li Hua, Director of Oncology at Jiahui International Hospital,
The emergence of CAR-T cell therapy has brought new hope to cancer patients worldwide. China’s CAR-T therapy offers outstanding efficacy, good safety, low cost, and rapid preparation advantages. Several international hospitals have opened green channels for overseas patients, making China the best treatment destination for global late-stage multiple myeloma patients!

References:
[1] Yuting Yan, et al. Blood Adv. 2019 Oct 8;3(19):2895-2904.[2] 2023 IMS. P-290.
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Breaking Barriers: Equecabtagene Autoleucel Revives Hope for International RRMM Patients – Multiple Myeloma
Breaking Barriers: Equecabtagene Autoleucel Revives Hope for International RRMM Patients – Multiple Myeloma

Multiple Myeloma
Relapsed/refractory multiple myeloma (RRMM) presents a significant challenge worldwide, representing 13% of all blood cancers. Despite advances in treatment, many patients face poor outcomes and limited options. However, a groundbreaking CAR-T therapy, Equecabtagene Autoleucel, has recently demonstrated its life-changing potential in overcoming this medical impasse.
In February 2024, a 70-year-old patient from Kyrgyzstan, diagnosed with multiple myeloma 17 years ago, arrived at a Hospital of Chinese Xi’an seeking relief from severe pain and disease progression. The patient had undergone multiple treatments over the years, including chemotherapy and radiotherapy, but his condition continued to deteriorate with extensive bone destruction and kidney disease caused by the cancer.
Given the severity of his condition and the failure of previous treatments, he was selected for Equecabtagene Autoleucel therapy. After a series of preparatory treatments, the patient received CAR-T cell infusion in April 2024, and the results were remarkable. Within just one month, his myeloma cells were undetectable, and he achieved a complete response (CR) with no signs of minimal residual disease (MRD). The treatment was not only effective but also safe, with only mild side effects such as grade 1 cytokine release syndrome (CRS), which was easily managed.
This case exemplifies the transformative power of Equecabtagene Autoleucel, which has shown a 98.9% overall response rate (ORR) in clinical trials, with a CR rate of over 82%. It offers a beacon of hope for RRMM patients, particularly those who have exhausted conventional treatment options.
As one of China’s premier CAR-T therapies, Equecabtagene Autoleucel is breaking barriers, providing a new lease on life for patients globally. With its impressive safety profile and unparalleled efficacy, it is poised to play a pivotal role in extending survival and improving quality of life for those battling RRMM. This therapy is not only revolutionizing cancer treatment but also proving to be a lifeline for patients who previously faced limited options.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#MultipleMyeloma #CARTTherapy #CancerTreatment #MedicalInnovation #GlobalHealthcare #HopeForPatients #ChinaMedicalBreakthroughs #EquecabtageneAutoleucel #CAR_T #RRMM
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Celebrating Discharge: The Joy of a New Life/ Hope /Multiple myeloma
Celebrating Discharge: The Joy of a New Life/ Hope /Multiple myeloma
After a period of treatment, the Singaporean patient Teresa achieved remarkable results with CAR-T therapy at Jiahui International Hospital in Shanghai. Her condition reached complete remission (CR), and to celebrate this great news, the hospital held a brief yet heartwarming celebration for her.
In the celebratory photos, Teresa, along with Dr. Vicky Lee and her team of doctors and nurses, are all smiles, radiating the joy of victory. The photos not only captured this happiness but also served as a testament to the hard work and professionalism of the medical staff. Every member of Dr. Vicky Lee’s team is a true hero, using their expertise and selfless dedication to help Teresa overcome her illness.
Teresa, filled with emotion, said: “Although the treatment process was tough, I felt immense warmth and support from the doctors and nurses at Jiahui Hospital. Their professionalism and care gave me confidence, which ultimately led to such a successful outcome.”
On the day of her discharge, the medical staff extended their heartfelt blessings to Teresa. The nurses kindly reminded her of the precautions she needed to take after leaving the hospital, ensuring she could maintain good health during her recovery at home. Teresa expressed her gratitude to each member of the medical team, thanking them for the care and support they provided when she needed it most.
Standing at the hospital entrance, Teresa looked back on her treatment journey, filled with gratitude and hope. She knew that it was because of the selfless dedication and outstanding professionalism of these medical professionals that she could embrace health once again and look forward to a new chapter in life.
Teresa’s recovery story is not only a personal victory but also the result of the collective efforts of the entire medical staff at Jiahui Hospital. She is deeply grateful for their hard work and believes that many more patients will find new hope for recovery here in the future.
We will continue to follow the patient’s post-treatment progress and provide updates.
#CART #CARTTherapy #Hopeforpatients #FUCASO #Equecel #MultipleMyeloma #jihuiHospital #Shanghai #ChineseCart #MedicalInnovation #MedicalBreakthrough #CancerTreatment #FullyHumanCART #cancerfight #cancersurvivor #Jiahuihospital
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Groundbreaking Results for Chinese CAR-T Therapy: 86% Response Rate of BCMA/GPRC5D Dual-Target Treatment Multiple myeloma
**Groundbreaking Results for Chinese CAR-T Therapy: 86% Response Rate of BCMA/GPRC5D Dual-Target Treatment**

Multiple myeloma

