Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Redefining Hope for High-Risk RRMM Patients Worldwide: A Singaporean Patient`s Journey to China for Groundbreaking CAR-T Therapy
Redefining Hope for High-Risk RRMM Patients Worldwide: A Singaporean Patient`s Journey to China for Groundbreaking CAR-T Therapy

Multiple Myeloma
#MultipleMyeloma #CAR_Therapy #CancerTreatment #HRMM #MM #RRMM #CART
In the fight against multiple myeloma (MM), the last few decades have seen significant advancements, yet the disease remains notoriously difficult to cure, particularly in patients with relapsed/refractory multiple myeloma (RRMM). These patients face enormous challenges, as their options become increasingly limited after multiple lines of therapy have failed. However, hope has emerged in the form of BCMA CAR-T therapy, offering deep remission and long-term survival for those who had nearly lost hope.
One such case is a 58-year-old woman from Singapore, who after exhausting all available treatments in her home country, found new hope in China`s innovative CAR-T therapy. Diagnosed with MM in May 2021 following a month of severe back pain, she underwent a series of treatments including CD38 monoclonal antibodies, immunomodulatory drugs (IMiDs), proteasome inhibitors (PI), and XPO-1 inhibitors. Unfortunately, these therapies failed to halt the progression of her disease, which had become highly resistant to treatment.
In December 2023, she traveled to Beijing Chaoyang Hospital, Capital Medical University, where Professor Chen Wenming took charge of her case. The patient was diagnosed with high-risk MM (IgG-κ type) and admitted to the hospital on November 25, 2023, for CAR-T therapy.
#### The Treatment Journey: A Detailed Overview
Given the patient’s refractory nature and multiple prior treatments, Professor Chen devised a tailored treatment plan to improve her chances of survival and quality of life. In November 2023, her lymphocytes were collected to prepare the CAR-T cells. During this period, she received two cycles of D-PACE (dexamethasone, cisplatin, doxorubicin, cyclophosphamide, and etoposide) chemotherapy in Singapore to control the extramedullary plasmacytoma.
In February 2024, she returned to Beijing for further evaluation, where her condition was assessed as showing minimal response (MR). She was then administered a lymphodepletion regimen of fludarabine and cyclophosphamide on February 29. The following month, she received a transfusion of BCMA CAR-T cells.
Within five days post-transfusion, the patient developed a fever, which peaked at 39°C. Fortunately, her oxygen saturation and heart rate remained normal, and she was diagnosed with Grade 1 cytokine release syndrome (CRS). After symptomatic treatment, her blood counts recovered by day 15, and she was discharged in stable condition.
Two weeks post-CAR-T therapy, her response was evaluated as a very good partial response (VGPR), and by the two-month mark, her condition had improved to complete remission (CR) with minimal residual disease (MRD) negativity.
#### Insights from Leading Experts
Professor Wee Joo Chng, a specialist in high-risk MM, noted the aggressive nature of the patient’s disease, marked by genetic abnormalities like 1q21+ and t(4;14). Despite the use of multiple potent therapies, including KRd, XVd, and Isa-Pd, the patient’s disease continued to progress rapidly. The emergence of the del(17p) mutation further complicated her prognosis, indicating the need for a novel therapeutic approach.
The FUMANBA-1 study has highlighted the effectiveness of China`s indigenous CAR-T product, Equecabtagene Autoleucel, in achieving deep remission and prolonging survival in RRMM patients. This case demonstrated the therapy`s potential to overcome poor prognostic factors and extend the patient’s survival. Notably, the patient experienced only mild CRS and no immune effector cell-associated neurotoxicity syndrome (ICANS) or infections during treatment. At the two-month follow-up, the patient’s condition had improved to CR with MRD negativity, suggesting that Equecabtagene Autoleucel could be a game-changer for high-risk RRMM patients.
#### A New Frontier in CAR-T Therapy
RRMM patients with double-hit characteristics often experience early relapse and progression, leading to shortened survival times. Traditional therapies, including IMiDs, PIs, and monoclonal antibodies, have failed to overcome these poor prognostic factors, indicating the urgent need for novel treatments. Real-world studies have shown that CAR-T therapy offers comparable progression-free survival (PFS) and overall survival (OS) rates in RRMM patients, regardless of high-risk cytogenetic abnormalities.
The FUMANBA-1 study revealed impressive outcomes for Equecabtagene Autoleucel in RRMM patients, with an overall response rate (ORR) of 98.9% and an MRD negativity rate of 97.8% among CAR-T-naive patients. The CR rate was 82.4%, and 81.7% of patients maintained MRD negativity for over a year.
Globally, four CAR-T products are currently available, and a recent study presented at the 2024 European Society for Blood and Marrow Transplantation (EBMT) compared the short- and long-term efficacy of these therapies. The study’s matching-adjusted indirect comparison (MAIC) analysis revealed that Equecabtagene Autoleucel had a 12-month PFS rate of 94.2%, higher than the 75% observed with Ciltacabtagene autoleucel (CARTITUDE-1 study). Furthermore, the 12-month sustained MRD negativity rate for Equecabtagene Autoleucel was 100%, compared to 53.1% for Ciltacabtagene autoleucel.
These findings suggest that Equecabtagene Autoleucel, a Chinese-developed BCMA CAR-T therapy, offers superior long-term efficacy compared to its U.S. counterpart. As the global community celebrates the first anniversary of its approval, Equecabtagene Autoleucel continues to bring hope to RRMM patients worldwide, further solidifying China’s leading role in the field of cellular therapy.

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp:+8613717959070
Email: doctor.huang@globecancer.com
#ChinaMedicalInnovation #GlobalHealth #MMResearch #PatientCare #InnovativeMedicine #CellTherapy #MedicalBreakthrough
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

22 Days from Desperation to Rebirth! Chinese CAR-T Therapy Creates Survival Miracle for Thai Multiple Myeloma Patient
22 Days from Desperation to Rebirth! Chinese CAR-T Therapy Creates Survival Miracle for Thai Multiple Myeloma Patient
Reference:
[1]Yuting Yan, et al. Blood Adv. 2019 Oct 8;3(19):2895-2904.
[2] 2023 IMS. P-290.
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Overcoming Challenges and Embracing New Life! – Breakthrough Progress of Eque-cel in the Treatment of Refractory Multiple Myeloma
### Overcoming Challenges and Embracing New Life! – Breakthrough Progress of Eque-cel in the Treatment of Refractory Multiple Myeloma

Multiple Myeloma
In the treatment of multiple myeloma (MM), how do we find new breakthroughs for patients who have not achieved complete remission (CR) after multiple rounds of chemotherapy? Research by Chinese medical professors has provided an exciting answer: Eque-cel (BCMA CAR-T therapy).
**Patient Background:**
This 58-year-old female patient was initially admitted to the hospital due to numbness and pain in both lower limbs and was eventually diagnosed with multiple myeloma. Despite receiving various treatment regimens, including VRD and SVPD, the results were unsatisfactory, and complete remission was not achieved. Faced with refractory characteristics, the doctors decided to try a more innovative treatment plan—CAR-T cell therapy.
**Treatment Process:**
In September 2023, the patient began peripheral blood mononuclear cell collection, followed by bridging therapy, and in November 2023, she received the Eque-cel infusion. Remarkably, just one month later, the patient achieved hematologic complete remission (CR) with minimal residual disease (MRD) negativity. After six months of follow-up, the patient maintained this excellent therapeutic effect.
**Professor’s Insights:**
Chinese medical professors pointed out that the advent of Eque-cel has brought new hope to refractory MM patients. The drug demonstrated significant efficacy in the FUMANBA-1 study: the overall response rate was as high as 98.9%, with 82.4% achieving complete remission, and 97.8% of patients achieving MRD negativity. The 12-month sustained MRD negativity rate reached 81.7%, and the PFS rate was 85.5%.
This outstanding result proves the significant advantage of Eque-cel in improving the depth of remission for MM patients, bringing hope for long-term survival to many refractory patients.
**Future Outlook:**
As the application and research of Eque-cel continue, we look forward to it providing better treatment options and survival opportunities for more MM patients. This new treatment plan is bringing a ray of hope to this stubborn disease and providing valuable experience for clinical experts worldwide.
**Stay Tuned:**
We will continue to follow the latest developments and research progress of Eque-cel, looking forward to its greater role globally, bringing hope and blessings to more patients.

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp:+8613717959070
Email: doctor.huang@globecancer.com
#MultipleMyeloma #CARTTherapy #MedicalBreakthrough #EqueCel #CancerTreatment #MedicalResearch #Hematology #PatientCare #OncologyInnovation #HopeForPatients #BCMA #CART #MM #RRMM
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

“Thank you,” Teresa said to the doctors and nurses with a smile, “Your professionalism and care make me feel incredibly reassured. I believe that with you by my side, I will conquer this illness.”
### Gratitude to the Doctors and Nurses at Jiahui International Hospital in Shanghai:
After undergoing the processes of apheresis and reinfusion, Teresa feels immense gratitude towards the doctors and nurses at Jiahui International Hospital in Shanghai. She has deeply experienced the professionalism and care of the medical staff.
Under the guidance of Dr. Vicky Lee and her team, the entire collection and infusion process became smooth and reassuring. Every doctor and nurse patiently explained each step, effectively prevented and assessed the risks that might occur after reinfusion, and constantly monitored her physical condition and emotional changes, providing meticulous care and comfort. They also offered tremendous psychological support. The warm words and determined eyes of the doctors and nurses gave Teresa strength during moments of uncertainty.
It is precisely the professionalism and compassion of these healthcare workers that filled Teresa with confidence and hope on her journey to fight the illness. She sincerely thanks all the medical staff at Jiahui Hospital. Their dedication and care have shown her the hope of recovery.
We will continue to follow up on the patient’s treatment progress and provide updates.
#CART #CARTTherapy #Hopeforpatients #FUCASO #Equecel #MultipleMyeloma #jihuiHospital #Shanghai #ChineseCart #MedicalInnovation #MedicalBreakthrough #CancerTreatment #FullyHumanCART #cancerfight #cancersurvivor #Jiahuihospital
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

“谢谢你们,”Teresa微笑着对医生和护士们说道,“你们的专业和关爱让我感到无比安心。我相信,有了你们,我会战胜这场病魔。”
感谢上海嘉会国际医院的医生护士:
在经历了单采,回输等过程后,Teresa对上海嘉会国际医院的医生和护士们心怀感激。她深深体会到了医护人员的专业和关怀。
在Dr. Vicky Lee及其团队的指导下,整个采集输注过程变得顺利和安心。每一位医生和护士都耐心解释每一个步骤,甚至对回输后会出现的风险都做了有效预防和评估,时刻关注她的身体状况和情绪变化,给予她无微不至的照顾和安慰。并且在心理上给予了莫大的支持。医生和护士的温暖话语和坚定目光,让Teresa在不安时找到了力量。
正是这些医护人员的专业和爱心,让Teresa在对抗病魔的路上充满了信心和希望。她由衷地感谢嘉会医院的全体医护人员,是他们的付出和关怀,让她看到了康复的希望。
我们将持续关注患者的治疗后续,并跟进报道。
#CART #CARTTherapy #Hopeforpatients #FUCASO #Equecel #MultipleMyeloma #jihuiHospital #Shanghai #ChineseCart #MedicalInnovation #MedicalBreakthrough #CancerTreatment #FullyHumanCART #cancerfight #cancersurvivor #Jiahuihospital
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese Medical Team: Long Survival and Significant Benefits with BCMA CAR-T Treatment for RRMM: A 5-Year Outlook
**Chinese Medical Team: Long Survival and Significant Benefits with BCMA CAR-T Treatment for RRMM: A 5-Year Outlook**

RRMM
Chimeric antigen receptor (CAR) T-cell therapy is one of the most promising new treatments for relapsed/refractory multiple myeloma (RRMM), but reports on its long-term efficacy and safety are limited. As early as 2022, Professor Du Juan’s team from the Department of Hematology at Shanghai Changzheng Hospital published a Phase I/II study demonstrating that patients with poor physical status could also benefit from CAR-T therapy. Recently, the team updated their findings with a five-year long-term follow-up, focusing on factors affecting long-term clinical benefits. The results were published in *Clinical Cancer Research*. The following summary of the study’s content is provided by *Cancer Information* for readers’ benefit.
### Evidence for Long-Term Efficacy and Safety of BCMA CAR-T Cell Therapy
#### Patient Characteristics
The study included 49 RRMM patients who had all received at least three prior lines of therapy before undergoing BCMA CAR-T cell treatment. At enrollment, 20 patients (40.82%) had poor physical status (ECOG performance status of 3-4), 42.86% had high-risk cytogenetic features, and 63.27% had received four or more lines of treatment. At the time of infusion, 79.59% had progressive disease. Among the patients with poor physical status, 30% had extramedullary disease (EMD), 45% had high-risk cytogenetic features, 70% had received four or more lines of treatment, and 80% had progressive disease after their last line of treatment.
#### Efficacy Evaluation of BCMA CAR-T Cell Therapy HDS269B
After a median follow-up of 59.0 months, the study showed an overall response rate (ORR) of 77.55%. The ORR was similar across patients with different ECOG scores. The median progression-free survival (PFS) was 9.5 months, and the median overall survival (OS) was 20.0 months. The five-year PFS and OS rates were 21.3% and 31.4%, respectively. For patients with ECOG scores of 0-2, the median PFS was 11.0 months, compared to 4.0 months for those with scores of 3-4 (P=0.18). The median OS was 41.8 months for ECOG 0-2 patients and 10.5 months for ECOG 3-4 patients (P=0.015).
Patients who had previously undergone four or more lines of therapy had significantly shorter PFS and OS compared to those who had received fewer than four lines (PFS: P=0.012; OS: P=0.0049). Among the 11 patients with EMD at enrollment, the ORR was 64% for those with EMD and 82% for those without EMD. However, median PFS and OS were notably shorter in patients with EMD (PFS: 3.0 months vs. 10.5 months, P=0.06; OS: 5.0 months vs. 24.0 months, P=0.03).
#### MRD-Negative Status and CAR-T Cell Persistence Indicate Better Long-Term Survival
Minimal residual disease (MRD) negativity was significantly associated with longer PFS and OS. In this study, MRD data were available for 22 patients on day 28 post-infusion, with 14 patients (63.64%) achieving MRD negativity (10^-4). These patients experienced significantly longer PFS and OS compared to MRD-positive patients. Similar associations were observed with MRD status at 3 and 6 months post-infusion.
The expansion of CAR-T cells was also closely related to clinical outcomes. Patients who achieved partial response (PR) or better had higher CAR-T cell peak levels. Patients without disease progression five years post-infusion had significantly higher CAR-T cell expansion peaks than those with progression. Additionally, the duration of CAR-T cell persistence correlated with longer PFS and OS, with patients having CAR-T cells persisting for ≥6 months, ≥12 months, ≥24 months, and ≥36 months showing significantly better PFS and OS than those without detectable CAR-T cells.
#### Controlled Safety Profile of BCMA CAR-T Cell Therapy HDS269B
No new CAR-T cell-related toxicities were observed during long-term follow-up. All patients experienced at least one adverse event (AE), with the most common long-term (≥28 days post-infusion) grade ≥3 AEs being hematologic in nature. No second primary malignancies or delayed immune effector cell-associated neurotoxicity syndrome (ICANS) were observed.
This study also included survival analysis, classifying patients by PFS and OS. The results indicated that ECOG 0-2 status, fewer than four prior therapies, and CAR-T cell persistence for ≥6 months were independently associated with longer survival.
### The Potential of BCMA CAR-T Therapy and the Need for Future Optimization
Through a five-year long-term follow-up of 49 RRMM patients, this study systematically evaluated the efficacy and safety of BCMA CAR-T cell therapy HDS269B. The findings suggest that poor physical status is not a contraindication for CAR-T therapy, thus broadening the indications for this treatment. While the results are encouraging, the study has some limitations, including its open-label, single-arm design and small sample size, which, combined with the long follow-up period, could lead to some patient attrition. Furthermore, despite the lack of new severe toxicities, long-term safety requires continued observation.
Overall, this study underscores the importance of BCMA CAR-T cell therapy in the treatment of RRMM and provides a crucial basis for exploring and applying CAR-T immunotherapy in the frontline treatment of multiple myeloma.

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +86137 1795 9070
Email: doctor.huang@globecancer.com
#CAR_Therapy #BCMACART #MultipleMyeloma #CancerResearch #LongTermSurvival #MedicalInnovation #Hematology #CancerTreatment #Immunotherapy #OncologyAdvances
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Blood Cancer Solution Including leukemia, lymphoma, multiple myeloma, and others.
 Blood Cancer Solution
Blood Cancer Solution
 Including leukemia, lymphoma, multiple myeloma, and others.
Including leukemia, lymphoma, multiple myeloma, and others.

Blood Cancer
#leukemia #lymphoma #multiplemyeloma
#Hematologic malignancies are a group of malignant diseases originating from hematopoietic cells, often affecting the bone marrow, blood, and various organs and tissues throughout the body. Common types of hematologic malignancies include leukemia, myelodysplastic syndromes, lymphomas, multiple myeloma, and myeloproliferative neoplasms.
The causes of these diseases are complex, involving genetic mutations, immune abnormalities, radiation exposure, contact with harmful chemicals, infections, and hereditary factors. Additionally, poor lifestyle habits, high levels of stress, and environmental factors can also increase the risk of developing these conditions.
With an aging population and advancements in medical technology, the incidence of hematologic malignancies has been rising globally. In China, the incidence and mortality rates of leukemia and lymphoma are now among the top ranks of all malignancies.
However, hematologic malignancies are not incurable. In recent years, the treatment methods for these diseases have seen significant progress. From traditional combination chemotherapy and radiotherapy to hematopoietic stem cell transplantation, monoclonal antibody therapy, antibody-drug conjugates, small molecule targeted therapies, and the latest immunotherapies, treatment options have become increasingly diverse and precise.
Combination chemotherapy remains a primary treatment for many hematologic malignancies, despite its significant side effects. The efficacy of these treatments cannot be ignored. Modern chemotherapy regimens are continually being refined, including the incorporation of new cytotoxic drugs and targeted therapies, as well as the use of monoclonal antibodies. Additionally, the appropriate use of antiemetics, hematopoietic growth factors, and anti-infective agents helps to mitigate adverse effects.
Hematopoietic stem cell transplantation continues to be one of the most effective treatments for certain hematologic malignancies. The development of this treatment in China has been rapid, with 170 registered transplant centers by 2020.
Monoclonal antibodies, often referred to as “biological missiles,” have a high degree of specificity and single biological activity. They have revolutionized the treatment of hematologic malignancies. Antibody-drug conjugates (ADCs) utilize monoclonal antibodies to accurately identify tumor cell markers, guiding the delivery of chemotherapy drugs for targeted treatment.
Small molecule targeted therapies work by interfering with specific genes or proteins to inhibit tumor cell growth and proliferation. Gleevec, the first small molecule targeted therapy, increased the five-year survival rate for chronic myeloid leukemia (CML) patients from 30% to 89%, marking a breakthrough in cancer treatment. Today, there are numerous small molecule targeted drugs available for the treatment of hematologic malignancies, including BCR-ABL inhibitors, BTK inhibitors, BCL-2 inhibitors, PI3K inhibitors, and XPO1 inhibitors, with many more drugs currently in clinical trials expected to become available soon.
Immunotherapy includes immune checkpoint inhibitors (such as PD-1/L1), cancer vaccines, cellular immunotherapies (such as #CART), and nonspecific immunomodulatory treatments. #CARTtherapy, in particular, has gained widespread attention as an emerging curative treatment. This approach involves extracting a patient’s T cells, modifying them outside the body to specifically recognize and attack tumor cells, and then reinfusing the modified T cells into the patient. This therapy has been successfully applied to various hematologic malignancies, including acute lymphoblastic leukemia, lymphomas, and multiple myeloma. The first patient treated with CAR-T therapy has been disease-free for 11 years.
In recent years, China has made significant advances in the treatment of hematologic malignancies. The establishment of the “Chinese Expert Consensus on the Diagnosis and Treatment of High-Risk Multiple Myeloma” and the presentation by Professor Huang He at the 2024 #EHA conference on targeting CD7 universal CAR-T therapy for T-cell acute lymphoblastic leukemia (T-ALL) and T-cell lymphoblastic lymphoma (T-LBL) have shown remarkable efficacy and safety. Additionally, exciting new data from the 2024 American Society of Clinical Oncology (#ASCO) annual meeting highlighted the efficacy of Relma-cel in treating relapsed/refractory large B-cell lymphoma (R/R LBCL), with a four-year overall survival rate (#OS) of 66.7%. Particularly noteworthy is the research on multiple myeloma, where the BCMA-targeted CAR-T therapy has demonstrated deep and lasting responses, with a complete response (#CR) rate of 82.4% and a 12-month progression-free survival (#PFS) rate of 85.5%.
With the continuous development of new treatments and the emergence of new drugs, hematologic malignancies in China are no longer considered incurable diseases. Through standardized, individualized, and precise treatments, many patients with hematologic malignancies can achieve long-term disease-free survival, and even a cure, returning to normal work and life. As medicine continues to advance, every life will continue to shine brightly!

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#HematologicMalignancies #LeukemiaAwareness #LymphomaResearch #MultipleMyeloma #BloodCancer #CancerResearch #CAR_Therapy #StemCellTransplant #Immunotherapy #TargetedTherapy #MonoclonalAntibodies #CancerTreatment #MedicalAdvancements #CancerSurvivor #HealthcareInnovation
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

แสงแห่งความหวังใหม่: การเดินทางเพื่อการรักษาข้ามพรมแดนของผู้ป่วยชาวไทย
**แสงแห่งความหวังใหม่: การเดินทางเพื่อการรักษาข้ามพรมแดนของผู้ป่วยชาวไทย**
ที่แผนกโลหิตวิทยา โรงพยาบาลตงจี้เซี่ยงไฮ้ ผู้อำนวยการหลี่ผิง แพทย์ผู้รับผิดชอบการรักษาคุณ P ผู้ป่วยชาวไทยสูงอายุ ได้ให้รายละเอียดเกี่ยวกับภาวะมะเร็งเม็ดเลือดขาวชนิดมัลติเพิลมัยอิโลมาและประวัติการรักษาของเธอ สถานการณ์ของคุณ P มีความผันผวนอย่างมาก เนื่องจากเธอได้รับการรักษาหลายครั้งในประเทศไทย แต่โรคกลับมาเป็นซ้ำอีก ในที่สุดเธอได้เลือกและเชื่อมั่นในการรักษาด้วย CAR-T ในประเทศจีน ด้วยความหวังสุดท้าย
ผู้อำนวยการหลี่กล่าวด้วยความซาบซึ้งว่า “คุณ P เป็นนักสู้ที่กล้าหาญ และความกล้าหาญของเธอทำให้ฉันรู้สึกซาบซึ้ง แม้ว่าการรักษาด้วย CAR-T จะมีผลข้างเคียงอยู่บ้าง แต่เรามั่นใจว่าด้วยการจัดการอย่างมีวิทยาศาสตร์และประสบการณ์ที่กว้างขวางของทีมของเรา เราสามารถให้การดูแลและการป้องกันที่ดีที่สุดแก่เธอได้” เธอย้ำว่า การใช้ยาที่มี CAR-T มนุษย์เต็มตัวอย่าง FUCASO ซึ่งมีภูมิคุ้มกันต่ำ ทำให้ความเสี่ยงของผลข้างเคียงลดลงอย่างมาก มอบความหวังในการหายขาดให้กับผู้ป่วยมากขึ้น
ผู้อำนวยการหลี่และทีมของเธอยังคงยืนหยัดอยู่ในแนวหน้าของการรักษา โดยใช้ประสบการณ์และความเชี่ยวชาญที่กว้างขวางเพื่อให้บริการทางการแพทย์ที่มีคุณภาพสูงสุดแก่ผู้ป่วยทุกคน พวกเขาไม่ได้เพียงแค่รักษาโรค แต่ยังรักษาความหวังและความฝันของทุกครอบครัว ในการต่อสู้กับโรคนี้ พวกเขายืนหยัดร่วมกับผู้ป่วย ทำงานร่วมกันเพื่อมุ่งสู่อนาคตที่สดใส
ให้เราร่วมกันติดตามการเดินทางสู่การฟื้นคืนชีพของเธอที่โรงพยาบาลตงจี้ต่อไป
#การรักษาCAR_T #ผู้ป่วยชาวไทย #โรงพยาบาลตงจี้เซี่ยงไฮ้ #มัลติเพิลมัยอิโลมา #กลุ่มอาการการปลดปล่อยไซโตไคน์ #การรักษามะเร็ง #นวัตกรรมการแพทย์ #การแพทย์ข้ามพรมแดน #HopeReborn #CARTherapy #TongjiHospital #Shanghai #FUCASO #Equecel #MultipleMyeloma #PatientJourney #MedicalBreakthrough #CrossBorderTreatment #CancerSurvivor
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Expert Perspective: Side Effects and Management of CAR-T Therapy for a Thai Patient
**Expert Perspective: Side Effects and Management of CAR-T Therapy for a Thai Patient**
At the Hematology Department of Shanghai Tongji Hospital, Dr. Li Ping, the chief physician for the elderly Thai patient Ms. P, provided a detailed overview of the patient’s multiple myeloma condition and treatment journey. After experiencing multiple treatments and relapses in Thailand, the patient ultimately chose and trusted CAR-T therapy in China. Dr. Li highlighted that the most common side effect is cytokine release syndrome (CRS), which manifests as fever, hypotension, and difficulty breathing. While most CRS cases are mild to moderate, severe CRS can be life-threatening. She also emphasized that through scientific management, the team’s extensive experience, and the low immunogenicity of the fully human CAR-T product FUCASO, the side effects of CAR-T therapy can be effectively controlled, offering the patient hope for a cure.
We will continue to follow up on this patient’s treatment progress and provide updates.
#CARTherapy #MultipleMyeloma #FUCASO #Equecel #TongjiHospital #Shanghai #MedicalInnovation #CancerTreatment #Hematology #PatientJourney #Immunotherapy
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
High Risk Multiple Myeloma Solution and Chinese Expert Consensus
**High Risk Multiple Myeloma Solution and Chinese Expert Consensus**

Multiple Myeloma
High risk multiple myeloma (HRMM) refers to patients with multiple myeloma whose overall survival is less than 2 to 3 years under current standard treatments.
In 2024, the Chinese Society of Clinical Oncology’s Multiple Myeloma Expert Committee and the Chinese Anti-Cancer Association’s Hematologic Oncology Committee, organized by relevant experts, developed the “Chinese Expert Consensus on the Diagnosis and Treatment of High Risk Multiple Myeloma (2024 Edition),” which was officially published in the *Chinese Journal of Hematology* in May 2024. This consensus defines HRMM, outlines high-risk factors and risk stratification systems, and provides key treatment recommendations for HRMM, aiming to improve the quality of life and prognosis for HRMM patients in China.
**Definition of HRMM**
There is currently no precise definition of HRMM. Referencing the International Myeloma Working Group (IMWG)’s definition, the Chinese Expert Committee considers HRMM patients as those with an overall survival (OS) of less than 3 years after receiving autologous hematopoietic stem cell transplantation (auto-HSCT) or less than 2 years if they have not received auto-HSCT. Patients with OS of less than 2 years after receiving auto-HSCT are classified as ultra-high risk multiple myeloma (UHRMM) patients.
**Prognostic Factors of HRMM**
The biological characteristics of MM tumor cells and treatment response are key determinants in identifying HRMM.
**Static Prognostic Factors of MM**
-
**Genetic High-Risk Factors:**
In the context of genetic high-risk factors, cytogenetic abnormalities are core indicators in the MM risk stratification system, but there is still some debate over the definition of high-risk cytogenetic abnormalities (HRCAs). The “National Comprehensive Cancer Network (NCCN) Guidelines (2024.v1)” indicate that the presence of multiple HRCAs correlates with a poorer prognosis. Fluorescence in situ hybridization (FISH) is currently the main genetic testing technique for MM, and next-generation sequencing can be performed if conditions allow (TP53 mutations have a significant impact on prognosis, while the effects of KRAS, NRAS, DIS3, BRAF, and FAM46C are less clear). All these genetic tests require enrichment and selection of plasma cells.
-
**Non-Genetic High-Risk Factors:**
Confirmed non-genetic prognostic factors include International Staging System (ISS) stage III, extramedullary disease excluding bone lesions, circulating plasma cells, high plasma cell proliferation index, elevated lactate dehydrogenase (LDH), frailty, renal insufficiency, and thrombocytopenia.
**Dynamic Prognostic Factors of MM**
-
**Duration of Initial Treatment Response:**
The duration of response to initial treatment is a crucial dynamic prognostic factor for MM. Patients who received auto-HSCT followed by maintenance therapy and experienced relapse/progression within less than 2 years are classified as HRMM; for those who did not receive auto-HSCT, relapse within less than 18 months after starting treatment also indicates HRMM. Functional high risk refers to MM patients without known genetic high-risk factors at diagnosis who experience early progression within 18 months after the start of treatment.
-
**Depth of Initial Treatment Response:**
The depth of response to initial treatment is another important dynamic prognostic factor for MM. Patients who achieve negativity for minimal residual disease (MRD) in both bone marrow and imaging studies have the best survival outcomes. Achieving MRD negativity can partially overcome the adverse prognosis associated with high-risk cytogenetics. Continuous dynamic MRD monitoring has greater clinical value than a single MRD result, as sustained MRD negativity for more than 12 months can translate into long-term survival.
The Expert Committee considers newly diagnosed MM to be classified as HRMM if any of the following criteria are met:
-
R-ISS stage III, extramedullary disease excluding bone lesions, presence of circulating plasma cells (plasma cell leukemia is defined as ≥5% plasma cells in peripheral blood), presence of one or more HRCAs [t(4;14), t(14;16), t(14;20), del(17/17p), 1q21 gain/amplification, del(1p32), TP53 mutation], although 1q21 gain alone does not define HRMM;
-
MM patients who have received auto-HSCT followed by maintenance therapy and experience relapse within less than 2 years from the start of treatment;
-
Patients who have not received auto-HSCT and experience relapse within less than 18 months from the start of treatment;
-
Functional high risk;
-
Extramedullary relapse/secondary plasma cell leukemia;
-
New occurrence of 1q21 gain/amplification and/or del(17/17p)/TP53 mutation at relapse.
**Treatment of HRMM**
**Principles of Treatment for Newly Diagnosed HRMM**
The standard treatment for HRMM has not yet been established. The overall treatment strategy includes:
-
Utilizing combination therapies with drugs that have different mechanisms of action;
-
Aiming to eradicate all tumor clones, with the goal of achieving and maintaining MRD negativity both inside and outside the bone marrow;
-
Implementing a treatment strategy that adjusts based on the effectiveness of the therapy;
-
Acknowledging that current treatment outcomes for HRMM are still unsatisfactory, and encouraging the exploration of experimental therapies.
**Treatment for Newly Diagnosed HRMM Suitable for Transplantation**
-
**Induction Therapy Before Transplantation:**
For HRMM, induction therapy with the RVd regimen as a bridge to auto-HSCT has not met expectations in terms of depth of response and long-term prognosis, and achieving MRD negativity is more challenging compared to standard-risk patients. Some studies with novel drug-modified regimens have shown that patients with one HRCA receiving the KRd regimen (carfilzomib, lenalidomide, and dexamethasone) sequentially followed by auto-HSCT achieved similar MRD negativity rates and progression-free survival (PFS) as standard-risk patients, with no statistically significant difference. Meta-analyses indicate that incorporating a CD38 monoclonal antibody as part of the treatment backbone in early-line therapy provides clinical benefits for patients with HRCAs. For UHRMM patients, more intensive treatment regimens, such as the Dara-VRdC regimen (OPTIMUM/MUKnine study) and the Dara+KTD-PACE regimen (TT7 study), can be considered.
-
**Auto-HSCT:**
Tandem transplantation involves performing a planned second auto-HSCT within 3–6 months after the first. It is recommended that HRMM patients collect sufficient hematopoietic stem cells for two auto-HSCTs during the first mobilization. Regardless of the response achieved after the first transplant, it is advised to perform the tandem transplant within six months. The conditioning regimen for both transplants typically includes high-dose melphalan.
-
**Consolidation Therapy:**
If tandem transplantation is not performed, the original induction regimen can be continued for consolidation therapy for an additional 2–4 cycles.
-
**Maintenance Therapy:**
For HRMM patients, maintenance therapy should consider a combination of proteasome inhibitors, immunomodulators, and CD38 monoclonal antibodies, in either dual or triple drug regimens. It is recommended to continue maintenance therapy until disease progression or intolerance.
-
**Allo-HSCT:**
The long-term efficacy of allo-HSCT remains debatable and should only be considered within the context of clinical trials and for select high-risk patients.
#HRMM #AutoHSCT #CancerTreatment #MultipleMyeloma #InductionTherapy #TandemTransplantation #ConsolidationTherapy #MaintenanceTherapy #ProteasomeInhibitors #Immunomodulators #CD38MonoclonalAntibodies #AlloHSCT #UHRMM #NovelTherapies
Expert Consensus on the Treatment of HRMM
①Induction Therapy: For pre-transplantation induction therapy in HRMM, it is recommended to use a regimen based on CD38 monoclonal antibodies combined with proteasome inhibitors and immunomodulators. Recommended regimens include: Dara+KRd, Isa+KRd, Dara+VRd, and Isa+VRd. For patients who cannot tolerate a four-drug regimen, the KRd regimen is an alternative. For patients with significant extramedullary involvement (soft tissue or peripheral blood), additional cytotoxic drugs and, if necessary, radiotherapy can be added.
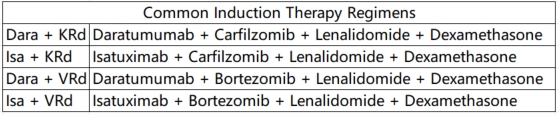
Therapy Regimens
②Auto-HSCT: Early auto-HSCT is the standard treatment for HRMM. For patients who receive auto-HSCT without significant adverse effects, tandem transplantation within six months post-transplant is recommended.
③Consolidation Therapy: For patients who do not undergo tandem transplantation, it is advised to continue consolidation therapy with the original induction regimen for 2–4 cycles.
④Maintenance Therapy: Maintenance therapy should involve a combination of proteasome inhibitors, immunomodulators, and CD38 monoclonal antibodies, either as dual or triple drug regimens. Therapy should continue until disease progression or intolerance.
⑤Clinical Research: Clinical studies targeting HRMM are encouraged, and it is recommended that HRMM patients prioritize enrollment in clinical trials.
**Treatment for Newly Diagnosed HRMM Not Suitable for Transplantation**
For MM patients not suitable for transplantation, an individualized treatment plan should be selected based on the patient’s fitness status score (IMWG GA score is recommended). For patients with good or moderate health, it is recommended to continue using the same regimen as for transplant-eligible patients. For frail patients, the VRd-lite (modified bortezomib + lenalidomide + dexamethasone) regimen and the DRd (daratumumab + lenalidomide + dexamethasone) regimen are currently the most commonly used first-line treatments.
**Treatment of Relapsed HRMM**
For patients with functional high risk and those defined as HRMM based on dynamic risk factors, re-induction regimens should include combinations of next-generation drugs or drugs with different mechanisms of action. Several clinical studies have shown that CAR-T cell (BCMA CAR-T) therapy can sustain efficacy in relapsed HRMM.
**Expert Consensus**
-
For relapsed HRMM patients, it is recommended to select combination regimens involving next-generation drugs or drugs with different mechanisms of action.
-
Patients with relapsed HRMM are encouraged to participate in clinical studies of CAR-T cell therapy or bispecific antibody immunotherapy.
**Summary**
For the treatment of HRMM, it is recommended to use multi-drug combination therapies with different mechanisms of action and bridge to auto-HSCT, aiming for deep and sustained MRD negativity and prolonging overall survival (OS) in patients. Currently, clinical trials of CAR-T cell therapy for newly diagnosed HRMM are being conducted, and combining auto-HSCT with CAR-T cell therapy can leverage the therapeutic benefits of both. Besides the BCMA target, CAR-T cell therapies targeting GPRC5D and FcRH5, as well as bispecific antibodies, have shown good efficacy in relapsed and refractory MM. New drugs and innovative diagnostic and therapeutic strategies, including immunotherapy, hold promise for overcoming the challenges in HRMM treatment.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#HighRiskMultipleMyeloma #HRMM #MultipleMyeloma #CancerTreatment #Hematology #Oncology #MedicalResearch #CancerCare #CancerAwareness #ChinaMedicalAdvances #AutoHSCT #MedicalConsensus #UHRMM #BloodCancer
#CancerPrognosis #GeneticRiskFactors #CancerResearch #MMTreatment #MRD #Hematology #CancerSurvival #PrognosticFactors
#CancerCriteria #Hematology #HighRiskMyeloma #PlasmaCellLeukemia #CancerRelapse #CytogeneticAbnormalities
#InductionTherapy #TandemTransplantation #ConsolidationTherapy #MaintenanceTherapy #ProteasomeInhibitors #Immunomodulators #CD38MonoclonalAntibodies #AlloHSCT #NovelTherapies
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Light of Hope – The Starting Point for Multiple Myeloma Patients
### Light of Hope – The Starting Point for Multiple Myeloma Patients
Ms. Teresa sat in the comfortable seat of the apheresis room, watching the nurse gently insert the needle into her arm to draw the precious white blood cell samples. These white blood cells would become the core material for CAR-T therapy, engineered and then reintroduced into her body to become a powerful force against cancer.
In the operating room, machines hummed softly, precisely separating the T cells from Teresa’s body. These cells, which had faithfully performed their duty of protecting her body, would now be re-educated to become a precise strike team against cancer cells. Teresa closed her eyes and silently prayed that this apheresis would infuse her body with the strength to drive the disease out of her life.
The apheresis process lasted several hours, with medical staff carefully monitoring each step to ensure enough high-quality cells were collected. The extraction of these cells was not only a technical challenge but also a stringent test of the medical team’s professional capabilities.
After the apheresis was completed, Teresa felt somewhat relieved. She knew this was just the first step in a long treatment journey, but it was also an important starting point filled with hope. In the apheresis room, she felt the warmth and professionalism of the medical team, which filled her with confidence and hope for the future treatment.
Back in her ward, Teresa gently closed her eyes as she lay on the bed. She pondered how the coming days would unfold, hoping that this apheresis would bring her the strength to overcome the disease and regain her health.
Apheresis, as the first step of the treatment journey, marked a new chapter in Teresa’s brave fight against cancer and ignited a flame of hope for her future recovery.
#ThePathOfHope #FightingCancer #JourneyOfCARTTherapy #TogetherWithTongji #FullyHumanCART #HopeWithFUCASO #EquecelApproval #HealthReborn #UnitedInPrayer #MultipleMyeloma
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

希望之光-多发性骨髓瘤患者的起点
