Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

2024 EBMT : China’s First RRMM CAR-T Therapy Equecabtagene Autoleucel: Efficacy Unaffected by Patients’ Baseline sBCMA Plasma Levels
2024 EBMT : China’s First RRMM CAR-T Therapy Equecabtagene Autoleucel: Efficacy Unaffected by Patients’ Baseline sBCMA Plasma Levels

RRMM
In recent years, CAR-T cell therapy targeting BCMA has emerged as a groundbreaking treatment for multiple myeloma, offering new hope to patients. At the 50th European Society for Blood and Marrow Transplantation (EBMT) Annual Meeting, held from April 14-17, 2024, in Glasgow, the team led by Professor Qiu Lugui presented the latest subgroup analysis results from the FUMANBA-1 study (Abstract OS10-04) on China’s first BCMA-targeted CAR-T therapy, Iquilencel (CT103A).
BCMA (B-cell maturation antigen) is a promising therapeutic target for multiple myeloma (MM), with soluble BCMA (sBCMA) levels in the blood reflecting tumor burden. High sBCMA levels can interfere with the effectiveness of BCMA-targeted therapies, including CAR-T, by competing with cell-surface BCMA for binding, which can lead to reduced efficacy. In contrast, Iquilencel has been designed to minimize the impact of sBCMA on treatment outcomes through careful selection of its single-chain variable fragment (scFv).
The FUMANBA-1 phase II study (NCT05066646) in Chinese patients with relapsed/refractory multiple myeloma (RRMM) has demonstrated that Iquilencel can induce deep and durable responses, with a complete response (CR) rate of 82.4% and a 12-month progression-free survival (PFS) rate of 85.5%. This study aimed to explore whether baseline serum sBCMA levels affect clinical outcomes following Iquilencel infusion.
### Study Methods and Results
The study used enzyme-linked immunosorbent assay (ELISA) to measure serum sBCMA levels and digital droplet PCR (ddPCR) to monitor CAR transgene copy numbers in patients’ peripheral blood. Baseline serum sBCMA levels were classified into high (≥225.1 ng/mL) and low (<225.1 ng/mL) groups. Results showed that high sBCMA levels were significantly associated with high tumor burden, advanced R-ISS and DS stages, and high BCMA expression. However, there were no significant differences in CAR-T cell expansion, AUC (Area Under the Curve) during the first 28 days, or cell persistence between the high and low sBCMA groups.
Patients with high baseline sBCMA levels had overall response rates (ORR) and ≥CR rates of 100% and 80%, respectively, compared to 97.8% and 84% in the low sBCMA group. Analysis showed no significant correlation between baseline characteristics (including sBCMA levels) and CR/sCR achievement. Additionally, there were no significant differences in minimal residual disease (MRD) negativity rates, 18-month sustained MRD negativity rates, PFS, and overall survival (OS) between the two groups.
### Conclusion
The findings from the FUMANBA-1 study indicate that Iquilencel’s efficacy is not influenced by baseline sBCMA levels, making it a universally applicable and promising treatment option for RRMM patients. Its unique fast-dissociation and low-exhaustion properties, similar to those of healthy T-cell receptors, enable Iquilencel to remain effective and persistent in patients’ bodies regardless of sBCMA levels.
Professor Qiu Lugui from the Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Sciences, and Professor Li Chunrui from Tongji Hospital, Huazhong University of Science and Technology, noted, “sBCMA is an important biomarker of tumor burden in multiple myeloma and a key factor influencing prognosis. Accumulation of sBCMA can inhibit the function of BCMA CAR-T cells. However, our study shows that Iquilencel can overcome the challenges posed by high baseline sBCMA levels, providing significant and lasting responses for RRMM patients.”
These results underscore Iquilencel as an ideal treatment choice for RRMM, offering hope for more effective and long-lasting therapeutic outcomes.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#EBMT2024 #CAR_T #MultipleMyeloma #Iquilencel #EquecabtageneAutoleucel #sBCMA #CancerResearch #Immunotherapy #MedicalBreakthrough #Biopharmaceuticals
Breakthrough medical research in China brings hope for multiple myeloma patients.
Eque-cel brings new treatment prospects for multiple myeloma.
From December 9th to 12th, the Annual American Society of Hematology Meeting (ASH 2023) was held in San Diego, USA. At this conference, the FUMANBA-1 study, led by Professors Chunrui Li from Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, and Lugui Qiu from the Institute of Hematology, Chinese Academy of Medical Sciences, conducted at 14 medical centers nationwide, demonstrated the efficacy and characteristics of sustained minimal residual disease (MRD) negativity in multiple myeloma (MM) patients treated with Eque-cel injection (CT103A). Their achievements were included in the oral presentation at the conference.

Current MM Treatment and Challenges of CAR-T Therapy CAR-T cell therapy holds significant importance in the treatment of multiple myeloma, overcoming patients’ immunodeficiency and tolerance issues. Currently, China has approved three CAR-T cell drugs targeting multiple myeloma. Compared to traditional treatments, CAR-T cell therapy enhances immune function without relying on the major histocompatibility complex (MHC), improving the activity of NK cells, T cells, and B cells while effectively activating T cells. However, despite significant advancements in CAR-T cell therapy in the MM field, some patients still struggle to maintain treatment effectiveness, leading to potential relapses, with antigen escape being one of the important mechanisms causing relapse.
Furthermore, the safety of CAR-T therapy is a major concern, including adverse reactions such as cytokine release syndrome (CRS), neurotoxicity, infections, B cell depletion, and hypogammaglobulinemia. Hence, monitoring patient indicators throughout the entire CAR-T treatment process, from pre-treatment to infusion, is crucial for timely intervention in adverse reactions.
100% MRD Negativity Rate for CR and Above Patients! Eque-cel Injection Brings Redemption for Multiple Myeloma
Eque-cel injection (CT103A) is a fully humanized CAR-T cell therapy drug targeting B cell maturation antigen (BCMA), approved by the National Medical Products Administration (NMPA) for adult refractory/relapsed multiple myeloma patients in China. In the Phase II FUMANBA-1 study, this treatment showed significant and sustained efficacy.
As of December 31, 2022, with a median follow-up time of 18.07 months, deep and sustained remissions were observed in 103 evaluable patients. The overall response rate (ORR) among these 103 patients was 96.1%, with a strict complete response (sCR)/complete response (CR) rate of 77.7%. Among 91 subjects with no previous CAR-T treatment history, the ORR was 98.9%, and the sCR/CR rate reached 82.4%, with a 12-month progression-free survival (PFS) rate of 85.5%.
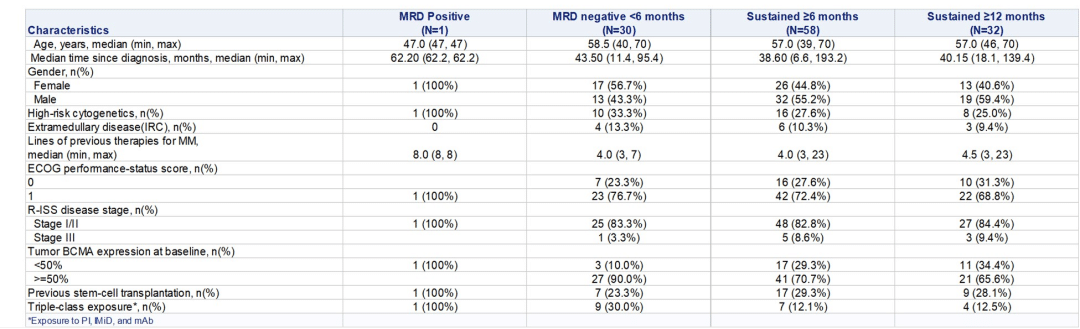
The minimal residual disease (MRD) negativity rate for the entire population was 94.2%, reaching 100% for CR and above patients. The median time to MRD negativity was 15 days, and 80.8% of patients maintained MRD negativity 12 months post-infusion. Moreover, the Eque-cel injection exhibited a relatively long median persistence in the body, lasting up to 307.5 days.
According to Professor Chunrui Li, “The sustained MRD-negative status is closely related to the improvement in PFS of patients receiving Eque-cel treatment.”
Professor Chunrui Li’s team studied the characteristics of patients maintaining MRD negativity for ≥6 months and ≥12 months in the FUMANBA-1 study. The research revealed that among 88 patients achieving MRD negativity, 78.4% maintained MRD negativity for at least 6 months, and 74.4% sustained MRD negativity for at least 12 months. Patients with sustained MRD negativity showed longer…

Professor Chunrui Li:
Chief Physician, Doctoral Supervisor
Secretary of the Hematology Department Party Committee, Deputy Director of Medicine Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology Professional
Focus: Immunotherapy for Hematological Malignancies
Member of the 11th Committee of the Plasma Cell Disease Professional Group of the Hematology Branch of the Chinese Medical Association
Chairman of the Expert Committee on Hematology (Hubei), Geriatrics Branch, Chinese Geriatrics and Gerontology Society
Standing Committee Member of the Geriatric Medicine Branch, Chinese Geriatrics and Gerontology Society
Youth Committee Member of the Oncology Branch, Chinese Anti-Cancer Association Committee Member of the Myeloma and Plasma Cell Disease Professional Group, 5th Committee of the Chinese Society of Clinical Oncology (CSCO) Leukemia Alliance & Lymphoma Alliance
Vice Chairman of the Blood Branch of the Hubei Medical Immunology Association Principal Investigator of four National Natural Science Foundation projects; published more than 20 SCI papers as first author or corresponding author, including Blood.
#EqueCelTreatment #MMResearch #CARTTherapy #MRDNegativity #CancerTreatment #MedicalBreakthrough #FUMANBA1Study #Immunotherapy #CancerResearchUpdate #CancerResearch #ASH2023 #BloodCancer #EqueCel #MRD #MultipleMyeloma
