Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

**Breaking News! China’s Cancer Drug Outperforms Global PD-1 Inhibitor!**
**Breaking News! China’s Cancer Drug Outperforms Global PD-1 Inhibitor!**

PD1
#AK112 #CancerDrug #PD-1 #PD1Inhibitor #CancerTreatment #BispecificAntibody #NSCLC
China’s pharmaceutical industry has reached a historic milestone! Remember AK112 (Ivonescimab), which took center stage during this year’s ASCO conference, attracting global attention for its groundbreaking potential? The long wait is finally over, and the results are in!
In May, Akeso Biopharma, a leading Chinese pharmaceutical company, announced that its independently developed bispecific antibody drug AK112 achieved a major milestone in clinical trials. AK112 demonstrated superior efficacy to the globally renowned PD-1 inhibitor Keytruda (K Drug) in treating PD-L1-positive non-small cell lung cancer (NSCLC), marking a revolutionary breakthrough for China’s innovative cancer treatments.
**Clinical Data that Surprised the World**
During the World Conference on Lung Cancer 2024, Professor Zhou Caicun, one of China’s top lung cancer experts, revealed the results of the HARMONi-2 study, which had the entire audience applauding. The data exceeded expectations, demonstrating that AK112 is not just on par with but superior to international “superstar” cancer drugs in several key areas.
– **Progression-Free Survival (PFS):** AK112 improved PFS by an astonishing 91.4% compared to Keytruda, reaching 11.14 months versus 5.82 months.
– **Risk Reduction:** AK112 reduced the risk of disease progression or death by 49%.
– **Objective Response Rate (ORR):** AK112 outperformed Keytruda with a response rate of 50.0% compared to 38.5%.
– **Disease Control Rate (DCR):** AK112 maintained a significant advantage, achieving 89.9% versus 70.5%.
While AK112 showed slightly higher Grade 3 side effects due to its VEGF inhibition, its overall performance remains remarkable. Although overall survival (OS) data is still maturing, AK112’s impressive PFS results suggest that its survival benefits will likely be just as extraordinary.
**The Power of Bispecific Antibodies**
AK112 is part of a new class of cancer drugs called bispecific antibodies, capable of targeting two different antigens at once. In this case, AK112 targets both PD-1 and VEGF, two well-known lung cancer markers. This dual-target approach offers patients better therapeutic options and increased convenience, potentially replacing the traditional combination of PD-1 inhibitors with anti-angiogenic drugs like Avastin.
This breakthrough has made AK112 the first and only bispecific antibody drug to outperform Keytruda in head-to-head clinical trials, solidifying its place as a future game-changer in cancer treatment.
**A Milestone for China’s Pharma Industry**
In May 2024, AK112 received regulatory approval from China’s National Medical Products Administration (NMPA) for treating EGFR-mutated, locally advanced, or metastatic NSCLC, becoming the world’s first approved PD-1/VEGF bispecific antibody.
This marks a monumental achievement for China’s pharmaceutical innovation. AK112’s success demonstrates that Chinese researchers and drug developers, in just two decades, have achieved what took developed nations over a century. China’s presence at top-tier global cancer conferences like ASCO and ESMO has never been stronger, with clinical data that leaves the world in awe.
China’s pharmaceutical industry may have started late, but its rapid progress is a testament to the ingenuity and hard work of its researchers. Once relying on generics, China is now a leader in pharmaceutical innovation, offering cutting-edge treatments to patients around the globe. The future of cancer treatment is here, and it’s painted in the bold color of “China Red.”
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CancerTreatment #MedicalInnovation #PharmaBreakthrough #ChinaPharma #AK112 #LungCancer #BispecificAntibody #PD1Inhibitor #GlobalHealth #MedicalAdvancements #ProudlyMadeInChina #WorldClass
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
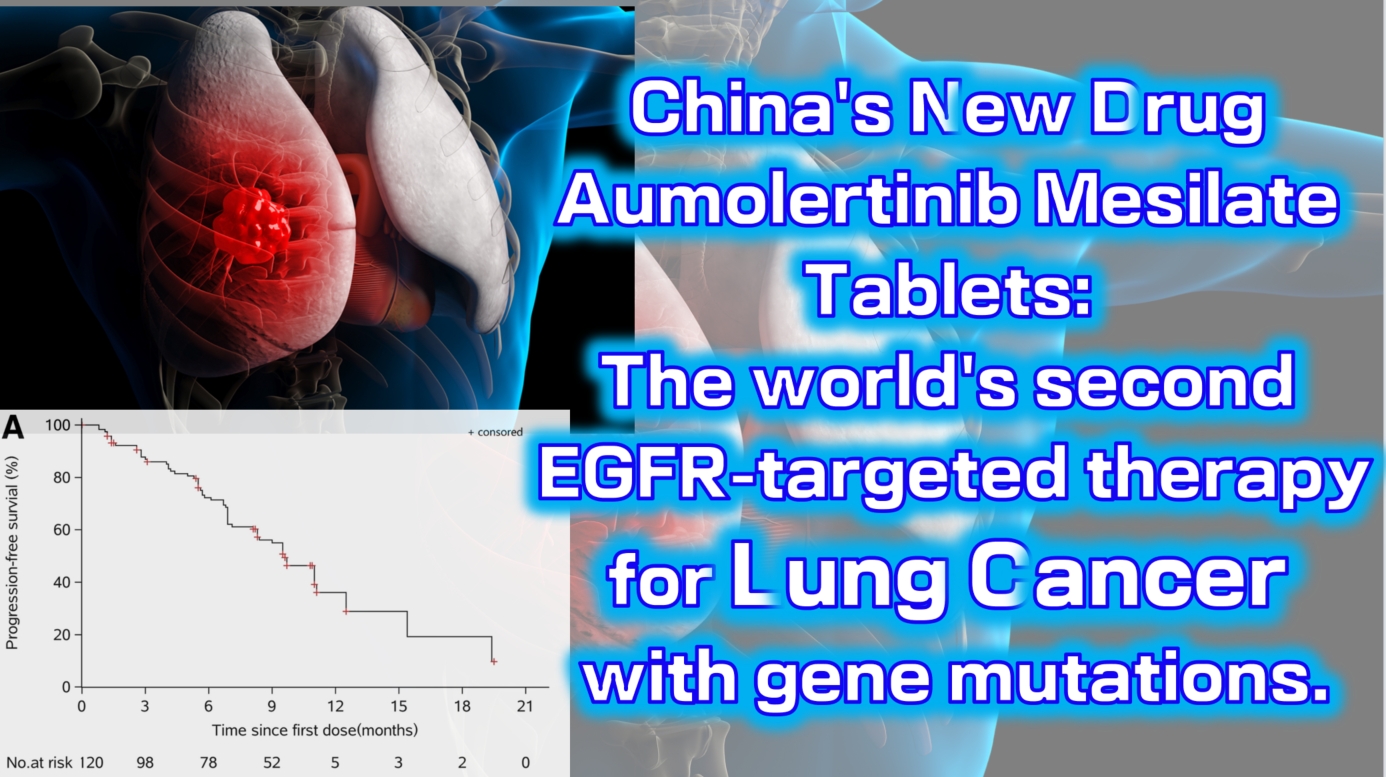
China’s new drug Aumolertinib Mesilate Tablets: The world’s second EGFR-targeted therapy for lung cancer with gene mutations.
China’s new drug Aumolertinib Mesilate Tablets: The world’s second EGFR-targeted therapy for lung cancer with gene mutations.

lung cancer
#LungCancer #EGFRInhibitor #TargetedTherapy #Aumolertinib #NSCLC
Aumolertinib Mesilate Tablets (Ameile) were approved for marketing in China on March 18, 2020. It is China’s first third-generation EGFR-targeted therapy for lung cancer with gene mutations and the second such drug globally, following Osimertinib.
On August 20, 2024, the new indication marketing application for Aumolertinib Mesilate Tablets was accepted for priority review by the China Center for Drug Evaluation (CDE). This indication is for the treatment of unresectable locally advanced non-small cell lung cancer (NSCLC) that has not progressed following platinum-based chemoradiotherapy. This is the fourth indication for which the drug has submitted a marketing application, and it is expected to be approved in the second quarter of 2025.
The Phase 1 clinical trial (NCT0298110) enrolled a total of 120 patients with locally advanced or metastatic non-small cell lung cancer (NSCLC). The results showed an objective response rate (ORR) of 52% (range: 42–63), with a disease control rate (DCR) as high as 92% (range: 84–96). The median progression-free survival was 11.0 months (95% CI: 9.5-not reached).

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#CancerTreatment #ClinicalTrials #PharmaInnovation #Oncology #DrugApproval #Biotech #PrecisionMedicine
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
The Hidden “Anti-Cancer Weapon” in Tumor Tissue – TIL Cells
### The Hidden “Anti-Cancer Weapon” in Tumor Tissue – TIL Cells

TIL Therapy
**TIL Cells: Turning Tumor Tissue “Waste” into Treasure**
Lymphocytes are a crucial part of the immune system. They can be found both in the periphery and within tumor tissues, serving as the body’s natural defense against tumor cells. Traditionally, only a small portion of excised tumor tissue is used for pathological diagnosis, while the majority is discarded as medical waste. This practice wastes the valuable anti-cancer resources hidden within the patient’s own body!
Tumor-Infiltrating Lymphocyte (TIL) therapy involves extracting lymphocytes from the tumor biopsy tissue of cancer patients. Using DNA sequencing technology, these lymphocytes surrounding the tumor cells are collected, expanded, and cultured in vitro before being reinfused into the patient. TIL therapy has shown positive clinical outcomes in various solid tumors, such as non-small cell lung cancer, melanoma, colorectal cancer, ovarian cancer, cervical cancer, and head and neck cancers. Compared to other T-cell therapies like CAR-T and TCR-T, TIL therapy offers broader targeting, more precise recognition, and more enduring anti-cancer effects.
**Applications of TIL Cells**
1. **For Early-Stage Cancer Patients:** TIL cells can be used as adjuvant therapy after surgery, helping to eliminate residual cancer cells following traditional treatments like surgery, radiotherapy, and chemotherapy, thereby preventing tumor recurrence or metastasis.
2. **For Advanced Cancer Patients:** TIL cells can serve as a last-line salvage treatment, aiding in extending survival time, improving quality of life, and addressing the dilemma of “no available drugs” for late-stage cancer patients.
**The Relationship Between TIL Cells and Cancer Patient Prognosis**
Using “non-small cell lung cancer” (#NSCLC) as an example, we can explore the impact of peripheral and tumor-infiltrating lymphocytes (TIL cells) on the prognosis of cancer patients.
A hospital in Tianjin, China, collected peripheral blood and tumor tissue samples from 105 newly diagnosed and untreated stage III/IV NSCLC patients. They analyzed the overall composition of peripheral blood cells and TIL cells, calculating and analyzing the overall survival (OS) of all patients. The results were as follows:
1. The median overall survival (mOS) for all patients was 12 months. The 1-year, 2-year, and 3-year overall survival (OS) rates were 60.5%, 28.4%, and 18.6%, respectively.
2. Patient OS was related to tumor size, tumor pathology, and CRP levels.
– **Tumor Size:** Patients with tumor lesions smaller than 4.8 cm had significantly longer OS compared to those with lesions larger than 4.8 cm (p=0.040).
– **Tumor Pathology:** Patients with squamous cell carcinoma had significantly longer OS compared to those with adenocarcinoma (p=0.025).
– **CRP Levels:** Patients with CRP levels below 8.29 mg/L had significantly longer OS compared to those with higher CRP levels (p=0.011).
– **Tumor CD4+ and CD8+ T Cell Infiltration:** Patients with higher levels of tumor CD4+ and CD8+ T cell infiltration had significantly longer survival compared to those with lower infiltration levels (p<0.0001 and p=0.011, respectively).
In conclusion, in newly diagnosed stage III/IV NSCLC patients, OS is associated with increased numbers of peripheral lymphocytes, CD4+ TIL, and CD8+ TIL, and is positively correlated with several basic clinical factors. This highlights the necessity of timely TIL cell storage. Patients are advised not to waste their valuable anti-cancer immune cells.
**Timing of TIL Cell Storage**
Generally, TIL cells should be stored as early as possible, ideally before receiving other treatments like radiotherapy or chemotherapy. At this stage, TIL cells have the strongest anti-cancer capability for the following reasons:
1. **TIL Cell Quantity:** TIL cell numbers gradually decrease as the tumor progresses. Clinical studies on TIL cell therapy for melanoma have found that T cell and NK cell numbers significantly decrease with tumor progression.
2. **Postoperative Adjuvant Radiotherapy and Chemotherapy:** These treatments not only kill cancer cells but also destroy immune cells and normal cells, reducing the anti-cancer efficacy of immune cells. Clinical studies on TIL cell therapy for cervical cancer have shown that CD4+ and CD8+ cell numbers significantly decrease in late-stage tumor TIL cells.
Therefore, patients are encouraged to seize their precious and unique opportunity for anti-cancer treatment. With the consent of their primary physician, they should preserve fresh tumor tissue samples preoperatively and entrust professional institutions with TIL cell isolation, culture, and cryopreservation. This can be used for postoperative adjuvant therapy to eliminate residual cancer cells, prevent tumor recurrence, or serve as a last-line salvage treatment for advanced cancer patients.
Since its initial clinical application in 1988, TIL therapy has developed over more than thirty years. Compared to other T-cell therapies, TIL therapy has the unique advantage of turning the patient’s own tumor tissue “waste” into a valuable resource, offering unique benefits in treating solid tumors and even helping some patients achieve long-term survival with the tumor.
China’s TIL therapy has been specially modified based on the American tumor-infiltrating lymphocyte (TIL) therapy to enhance TIL cell self-expansion ability and overcome the tumor microenvironment. In recent years, several new TIL therapies have been developed in China. Notably, the two TIL therapies showcased at the 2024 ASCO conference not only demonstrated impressive efficacy but also featured one therapy that requires neither lymphodepletion nor IL-2 treatment, significantly improving safety and cost-effectiveness!
The good news is that several TIL therapies have been officially approved for clinical research in China, targeting various solid tumors such as non-small cell lung cancer, cholangiocarcinoma, esophageal cancer, cervical cancer, ovarian cancer, and breast cancer. Patients seeking to store tumor tissue or seek TIL therapy assistance can consult Advanced Medicine in China
WhatsApp: 137 1795 9070
Email: doctor.huang@globecancer.com
#til #tilcell #cancer #nonsmallcelllungcancer #cholangiocarcinoma #carcinoma #esophagealcancer #cervicalcancer #ovariancancer #breastcancer #tumor #Tiltherapy #ASCO #asco2024 #2024asco
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
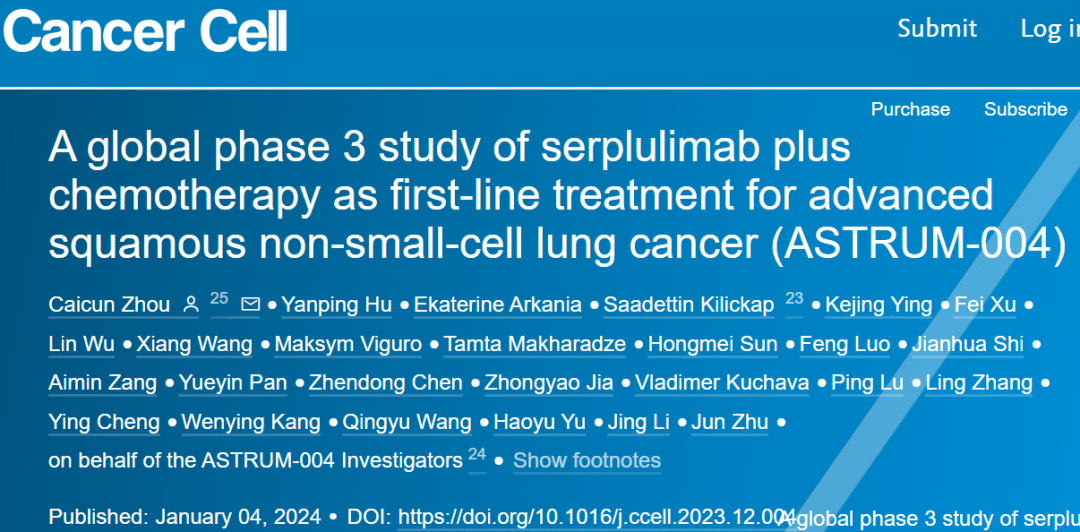
A New Era in Lung Cancer Treatment – Serplulimab Brings Innovative and Reliable Therapeutic Options!
**🌟 A New Era in Lung Cancer Treatment – Serplulimab Brings Innovative and Reliable Therapeutic Options! 🌟**

Cancer cell
📆CANCER CELL
January 5, 2023: The highly anticipated Phase 3 pivotal clinical trial (ASTRUM-004) of HANSIZHUANG, an anti-PD-1 monoclonal antibody developed independently by Henlius, in combination with chemotherapy for first-line treatment of advanced squamous non-small cell lung cancer (sqNSCLC), has just been published in the prestigious international oncology journal “Cancer Cell” (Impact Factor: 50.3)! Led by Professor Zhou Caicun from Shanghai Pulmonary Hospital, this milestone study signifies a significant breakthrough in lung cancer treatment.
🔬 Lung cancer
is one of the most common cancers globally, with the highest reported incidence and mortality rates in China. According to the latest statistics from the National Cancer Center, there are 828,000 new cases of lung cancer and 657,000 deaths annually. Non-small cell lung cancer (NSCLC) accounts for approximately 80% to 85% of all lung cancers, with a significant proportion diagnosed at an advanced stage or with metastasis, unsuitable for surgical resection.
🌱NSCLC
In the field of NSCLC treatment, immune checkpoint inhibitors have become a game-changer. Serplulimab is the first innovative monoclonal antibody developed independently by Henlius. Since its approval in March 2022, it has been approved in China for the treatment of microsatellite instability-high (MSI-H) solid tumors, sqNSCLC, extensive-stage small cell lung cancer (ES-SCLC), and esophageal squamous cell carcinoma (ESCC), benefiting over 51,000 patients.
🔍 ASTRUM-004
is a pivotal Phase 3 trial aimed at evaluating the efficacy and safety of serplulimab in combination with chemotherapy as first-line treatment for advanced sqNSCLC. The results are promising: patients receiving serplulimab demonstrated significantly prolonged progression-free survival (PFS) and overall survival (OS), with manageable safety profiles.
Serplulimab
🎉NEW ERA
These findings herald a new era in lung cancer treatment, offering a ray of hope for patients and clinicians alike. Serplulimab in combination with chemotherapy has emerged as a promising first-line treatment option, bringing innovation and reliability to sqNSCLC patients.

👩🔬Lead
Lead investigator Professor Zhou Caicun commented, “The ASTRUM-004 study validates the significant potential of serplulimab in combination with chemotherapy to improve survival outcomes in sqNSCLC patients, marking a major breakthrough in the field of lung cancer.”
🌟 Stay tuned for more updates as we continue to unravel the mysteries of cancer treatment, bringing hope to millions worldwide!
#CancerResearch #LungCancer #MedicalBreakthrough #InnovationForHealth #Serplulimab #NSCLC #ASTRUM #sqNSCLC #cancercell
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese Exciting Good News Update in Lung Cancer Treatment! ORR: 78.6%
 Chinese Exciting Good News Update in Lung Cancer Treatment! ORR: 78.6%
Chinese Exciting Good News Update in Lung Cancer Treatment! ORR: 78.6%
 Meet Sunvozertinib, the game-changer in advanced lung cancer therapy!
Meet Sunvozertinib, the game-changer in advanced lung cancer therapy! 

Sunvozertinib, NSCLC
At the 2023 ESMO Conference, fresh data from early trials of Sunvozertinib’s frontline treatment for EGFR exon20ins-mutated advanced NSCLC were unveiled, and the results were jaw-dropping! 
 With an astounding Objective Response Rate (ORR) of 78.6% and a median Progression-Free Survival (PFS) of 12.4 months at the recommended dose (300mg QD), Sunvozertinib once again set a new benchmark in this field, offering a superior treatment option for newly diagnosed patients with EGFR exon20ins mutations!
With an astounding Objective Response Rate (ORR) of 78.6% and a median Progression-Free Survival (PFS) of 12.4 months at the recommended dose (300mg QD), Sunvozertinib once again set a new benchmark in this field, offering a superior treatment option for newly diagnosed patients with EGFR exon20ins mutations! 

Let’s dive into a real success story: A 35-year-old male diagnosed with late-stage NSCLC harboring EGFR exon20ins mutations, along with metastases to the brain, liver, and bones. After showing stable disease (SD) following two cycles of platinum-based chemotherapy, he switched to Sunvozertinib. Just one month into treatment, a follow-up chest CT scan revealed a remarkable 34% reduction in the size of the left lower lobe lesion, with lung lesions shrinking from 29mm×26mm to 19mm×15mm (see image). Multiple nodules in the lungs and pleura also decreased in size, along with liver lesions. Evaluation: Partial Response (PR)! 


Lung Cancer
Noteworthy is the impeccable safety profile of Sunvozertinib, with no significant adverse effects reported during treatment. As of this post, the patient continues to receive Sunvozertinib monotherapy with promising outcomes! 

With breakthroughs in targeted therapies for EGFR exon20ins-mutated NSCLC, several new drugs are actively exploring frontline treatment options. The early study results of Sunvozertinib’s frontline therapy for EGFR exon20ins-mutated advanced NSCLC have once again rewritten the records in this field, providing new treatment guidelines for these patients! 

Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China’s Breakthrough: Sunvozertinib Leads the Wave of New Drug Development in EGFR exon20ins Mutant NSCLC
🌈🌈China’s Breakthrough: Sunvozertinib Leads the Wave of New Drug Development in EGFR exon20ins Mutant NSCLC🌸🌸
**Caption:**
🇨🇳 Exciting news from China’s research! Sunvozertinib, a novel drug by Dizal Pharmaceuticals, is making strides in treating EGFR exon20ins mutant NSCLC. 🌐✨

Sunvozertinib
**Article:**
Dizal Pharmaceuticals’ EGFR tyrosine kinase inhibitor (TKI) Sunvozertinib, known as 舒沃替尼 (Shuwo Tini) in China, has achieved a significant milestone with the publication of data from its Chinese registration study (WU-KONG6) in “The Lancet Respiratory Medicine.” This study validates the clinical efficacy of the Chinese-developed drug in treating late-stage non-small cell lung cancer (NSCLC) with EGFR exon20ins mutations, marking a crucial breakthrough for patients who have long faced challenges in finding effective treatments.
**Challenges in Treating EGFR exon20ins Mutant NSCLC**
EGFR exon20ins mutation has been a formidable subtype in EGFR-mutant NSCLC, with limited effectiveness observed in traditional EGFR-TKI and chemotherapy treatments. Faced with this challenge, pharmaceutical companies are actively engaged in new drug development to propel advancements in the treatment landscape for EGFR exon20ins mutant NSCLC. The success of the WU-KONG6 study signifies a critical recognition in this field and affirms the high efficacy and low toxicity of Sunvozertinib in treating EGFR exon20i

Lancet
ns mutant NSCLC.
**Results from the Chinese Registration Study “WU-KONG6″**
The results of the Chinese registration study “WU-KONG6” demonstrate that Sunvozertinib achieved an objective response rate (ORR) of 60.8% in treated patients with EGFR exon20ins mutations. This indicates a significant tumor reduction in over 60% of patients, leading to a substantial decrease in tumor burden. Moreover, patients experienced improvements in clinical symptoms and quality of life. Notably, Sunvozertinib is currently the only drug that has elevated the ORR in treated patients with EGFR exon20ins mutations to over 50%, breaking previous treatment ceilings and showcasing its potential as the “best in class.”
**Looking Ahead**
The competition in the field of EGFR exon20ins mutant NSCLC treatment is intensifying, and the successful introduction of Sunvozertinib provides a new therapeutic option for patients. As other pharmaceutical companies globally advance in new drug development, the innovation and competition in this field are expected to escalate, bringing more hope to patients with EGFR exon20ins mutant NSCLC. In the continual pursuit of medical breakthroughs, the emergence of new drugs will offer more effective treatment options, contributing to the extension of patients’ survival periods.
#MedicalInnovation #CancerResearch #HopeForPatients #Sunvozertinib #MedicalBreakthrough #ChinaInnovation
