Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
The Lancet | Chinese Medical Team: A New Breakthrough in the Treatment of Limited-stage Small Cell Lung Cancer!
The Lancet | Chinese Medical Team: A New Breakthrough in the Treatment of Limited-stage Small Cell Lung Cancer!
High-dose Accelerated Hyperfractionated Radiotherapy Helps Improve Treatment Efficacy

Lung Cancer
Small-cell lung cancer (SCLC) is a highly aggressive malignancy, accounting for 15% of all lung cancer cases. Among these, one-third are diagnosed with limited-stage SCLC (LS-SCLC), where the disease is confined to one side of the chest. For the past two decades, the standard treatment for LS-SCLC has been concurrent chemoradiotherapy, yet patients have seen little improvement in survival outcomes, with median survival ranging from 25-30 months.
A New Hope for LS-SCLC Patients
A recent study led by a team of Chinese researchers has shed light on a new treatment strategy. Published in The Lancet Respiratory Medicine, this multicenter, open-label, randomized phase III trial demonstrated that a high-dose, accelerated, hyperfractionated thoracic radiotherapy (54 Gy/30 fractions) combined with chemotherapy significantly improves overall survival (OS) and progression-free survival (PFS) for LS-SCLC patients. Importantly, this breakthrough treatment does not increase toxicity compared to the standard 45 Gy/30 fractions regimen.
Study Design and Methodology
The trial, conducted between 2017 and 2021, enrolled 224 patients across 16 hospitals in China. The patients, aged 18-70, had histologically or cytologically confirmed LS-SCLC and had either not yet received treatment or had undergone 1-2 cycles of chemotherapy. After chemotherapy, they were randomly assigned to receive either the high-dose (54 Gy/30 fractions) or standard-dose (45 Gy/30 fractions) thoracic radiotherapy, delivered twice daily over three weeks. Both groups also underwent four cycles of chemotherapy and, if responsive, prophylactic cranial irradiation (PCI) to prevent metastasis.
Improved Survival Outcomes
The study’s results were groundbreaking. Patients in the high-dose group showed a remarkable improvement in OS, with a median survival of 60.7 months, compared to 39.5 months in the standard-dose group. Additionally, the high-dose group saw a 45% reduction in the risk of death. Two-year survival rates also favored the high-dose group, with 76% of patients alive at two years, compared to 54% in the standard-dose group.
Progression-free survival was similarly improved, with the high-dose group experiencing a median PFS of 30.5 months, significantly longer than the 16.7 months observed in the standard-dose group. This translates to a 30% reduction in the risk of disease progression or death.
Safety and Tolerability
Despite the higher radiotherapy dose, the incidence of adverse effects between the two groups was comparable. The most common severe side effects were neutropenia, thrombocytopenia, and anemia, which occurred at similar rates in both groups. The occurrence of acute radiation-induced toxicity, such as esophagitis and radiation pneumonitis, also showed no significant differences. Importantly, there were no cases of severe late-stage toxicity, such as grade 3 esophageal stenosis or pulmonary fibrosis.
Clinical Implications and Future Directions
This study demonstrates that high-dose, accelerated hyperfractionated thoracic radiotherapy can significantly improve the prognosis of LS-SCLC patients without increasing treatment toxicity. It provides a new first-line treatment option that offers hope for longer survival. These findings may shift the clinical landscape, offering a viable alternative to the current standard of care.
As research continues to explore combining this high
A groundbreaking study led by a Chinese medical team has demonstrated that high-dose accelerated hyperfractionated chest radiotherapy (54 Gy/30 fractions) combined with chemotherapy significantly improves the overall survival (OS) of patients with limited-stage small-cell lung cancer (LS-SCLC). The research was published in The Lancet Respiratory Medicine on August 12, 2024. Compared to the standard dose of 45 Gy/30 fractions, the 54 Gy regimen increased OS without raising treatment toxicity, marking a potential shift in LS-SCLC treatment strategy.
Key Findings of the Study
LS-SCLC, a highly aggressive form of lung cancer, affects around 15% of all lung cancer patients, with one-third of them diagnosed at a limited stage. Traditionally, concurrent chemoradiotherapy (CRT) has been the preferred treatment option for LS-SCLC, yet the median survival rate has remained stagnant at around 25-30 months over the past two decades. The new study, however, highlights the efficacy of using higher doses of radiation to improve outcomes.
This phase III clinical trial was conducted across 16 hospitals in China between June 2017 and April 2021, involving 224 LS-SCLC patients. The trial compared two groups: a high-dose radiotherapy group (54 Gy/30 fractions twice daily) and a standard-dose group (45 Gy/30 fractions twice daily). Patients were treated with four cycles of chemotherapy and monitored through regular follow-ups. The results were remarkable.
Overall Survival: The high-dose group had a significantly extended median OS of 60.7 months compared to 39.5 months in the standard-dose group, reducing the risk of death by 45%.
Progression-Free Survival (PFS): The median PFS was also improved in the high-dose group, standing at 30.5 months compared to 16.7 months in the standard-dose group, with a 30% reduction in the risk of disease progression or death.
Safety: Despite the higher radiation dose, there was no increase in severe treatment-related side effects, such as neutropenia, thrombocytopenia, or radiation-induced pneumonia. Both groups exhibited similar rates of acute and long-term radiation toxicity.
Why Is This Study Important?
The study’s findings suggest that 54 Gy/30 fractions, delivered twice daily, may become a new standard of care for LS-SCLC patients. This approach significantly extends survival while maintaining a similar safety profile to the current standard dose. Moreover, with a two-year OS rate of 76% in the high-dose group, compared to 54% in the standard-dose group, the potential for long-term remission appears much greater.
A New Era in LS-SCLC Treatment?
While the current trial focused on radiotherapy and chemotherapy alone, future studies could explore combining this high-dose radiotherapy with immunotherapy. Recent breakthroughs, such as the ADRIATIC trial, have shown that immunotherapy following CRT can further improve survival outcomes for LS-SCLC patients. However, the safety of combining high-dose radiotherapy with immunotherapy needs further investigation due to potential risks like radiation-induced pneumonitis.
In conclusion, this study has paved the way for a new treatment option in LS-SCLC, offering hope for improved survival rates without additional toxicity. It marks a significant milestone in the battle against this aggressive form of lung cancer, providing a valuable alternative to the long-standing standard treatments.

 To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#LungCancerTreatment #SCLCTherapy #CancerResearch #MedicalBreakthrough #ChinaHealthcare #Oncology #LungCancerSurvival #Radiotherapy #CancerCare #ClinicalTrials
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Real-World Performance of China’s Anti-Cancer Drug Zanubrutinib in CLL/SLL Treatment: Efficacy and Safety
**Real-World Performance of China’s Anti-Cancer Drug Zanubrutinib in CLL/SLL Treatment: Efficacy and Safety**

CLL
#Ibrutinib #Zanubrutinib #CLL #SLL #BTKinhibitor #Oncology #cancerdrug #BTK #ASCO
With the advent of BTK inhibitors, the treatment paradigm for chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL) has undergone a complete transformation, moving away from traditional chemotherapy. Zanubrutinib, a powerful BTK inhibitor developed independently by BeiGene in China, is a next-generation Bruton’s tyrosine kinase inhibitor (BTKi) that has demonstrated remarkable efficacy in clinical trials. A study presented at the 2024 ASCO conference sheds light on the real-world treatment patterns and outcomes of zanubrutinib, providing valuable insights for patients and clinicians.
### **Study Background**
Zanubrutinib, a fully China-developed anti-cancer drug, launched in late 2019, has been shown to be more effective than the first-generation BTKi, ibrutinib, in CLL/SLL patients. This retrospective study analyzed patients treated with zanubrutinib between 2018 and 2023 at Kaiser Permanente Northern California, focusing on treatment patterns, adverse events (AEs), and survival outcomes in a real-world setting.
### **Study Findings**
The study analyzed 281 patients with key findings as follows:
– **Patient Profile**: The median age of patients was 71 years, with 64% being male. Most patients were white (75%), followed by African Americans (10%). Of the total, 190 patients switched from ibrutinib to zanubrutinib, while 91 patients were treated solely with zanubrutinib.
– **Adverse Events**: Whether patients switched from ibrutinib or started directly on zanubrutinib, the latter showed significantly lower rates of cardiac toxicity and treatment-limiting adverse events (TLAEs). The most common AEs for zanubrutinib included cytopenia and rashes/bruising, while ibrutinib was more often associated with atrial fibrillation and fatigue.
– **Dose Adjustments and Treatment Continuity**: Some patients adjusted their doses due to mild AEs, but the majority (79%) were still receiving zanubrutinib at the end of the study. While 13 patients died, there were no treatment-related deaths.
### **Study Conclusion**
This study demonstrates that regardless of prior ibrutinib use, China’s homegrown anti-cancer drug, zanubrutinib, exhibited excellent efficacy and safety. Compared to ibrutinib, zanubrutinib had a lower incidence of cardiac toxicity and maintained its effectiveness even in older patients with more comorbidities. These real-world data reinforce zanubrutinib’s pivotal role in the treatment of CLL/SLL, further validating its clinical application.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CancerTreatment #RealWorldData #CancerResearch #BeiGene #ASCO2024
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
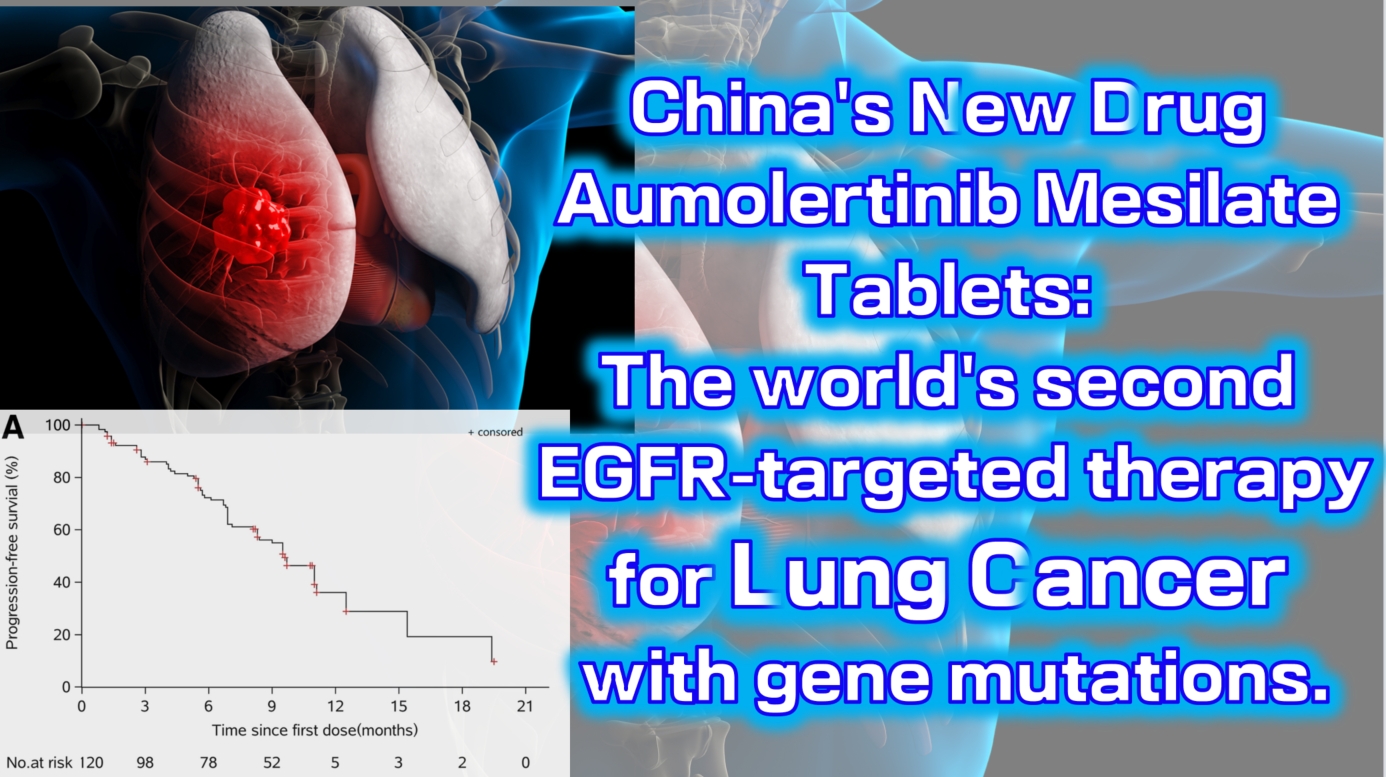
China’s new drug Aumolertinib Mesilate Tablets: The world’s second EGFR-targeted therapy for lung cancer with gene mutations.
China’s new drug Aumolertinib Mesilate Tablets: The world’s second EGFR-targeted therapy for lung cancer with gene mutations.

lung cancer
#LungCancer #EGFRInhibitor #TargetedTherapy #Aumolertinib #NSCLC
Aumolertinib Mesilate Tablets (Ameile) were approved for marketing in China on March 18, 2020. It is China’s first third-generation EGFR-targeted therapy for lung cancer with gene mutations and the second such drug globally, following Osimertinib.
On August 20, 2024, the new indication marketing application for Aumolertinib Mesilate Tablets was accepted for priority review by the China Center for Drug Evaluation (CDE). This indication is for the treatment of unresectable locally advanced non-small cell lung cancer (NSCLC) that has not progressed following platinum-based chemoradiotherapy. This is the fourth indication for which the drug has submitted a marketing application, and it is expected to be approved in the second quarter of 2025.
The Phase 1 clinical trial (NCT0298110) enrolled a total of 120 patients with locally advanced or metastatic non-small cell lung cancer (NSCLC). The results showed an objective response rate (ORR) of 52% (range: 42–63), with a disease control rate (DCR) as high as 92% (range: 84–96). The median progression-free survival was 11.0 months (95% CI: 9.5-not reached).

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#CancerTreatment #ClinicalTrials #PharmaInnovation #Oncology #DrugApproval #Biotech #PrecisionMedicine
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China’s first BCMA CAR-T therapy successfully treats an overseas patient with advanced relapsed multiple myeloma
China’s first BCMA CAR-T therapy successfully treats an overseas patient with advanced relapsed myeloma.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp:+8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#MultipleMyeloma #CAR-T #CancerTreatment #MedicalBreakthrough #Oncology #HealthcareInnovation #BCMACART
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Groundbreaking Results for Chinese CAR-T Therapy: 86% Response Rate of BCMA/GPRC5D Dual-Target Treatment Multiple myeloma
**Groundbreaking Results for Chinese CAR-T Therapy: 86% Response Rate of BCMA/GPRC5D Dual-Target Treatment**

Multiple myeloma
Multiple myeloma (MM) is a challenging blood cancer, particularly in relapsed/refractory (R/R) cases. While therapies like proteasome inhibitors, immunomodulators, CD38 monoclonal antibodies, and stem cell transplants have improved outcomes for newly diagnosed patients, treatment-resistant forms of MM remain a serious concern. A new therapeutic approach combining two targets, BCMA and GPRC5D, may provide a breakthrough solution.
BCMA has long been a key target for treating R/R MM due to its expression in malignant plasma cells. However, BCMA-targeted therapies face limitations, including tumor cells losing or downregulating BCMA, leading to disease recurrence. This is where GPRC5D, a protein highly expressed in MM cells and linked to poor prognosis, comes in. By targeting both BCMA and GPRC5D, researchers aim to overcome the limitations of single-target therapies.
A collaboration between a leading Chinese hospital and research institutions has now delivered promising results. A Phase I clinical trial involving 21 patients with R/R MM using the dual-target BCMA/GPRC5D CAR-T cell therapy reported an overall response rate (ORR) of 86%, with 75% of patients achieving a complete response (CR). Notably, even patients whose cancer cells lacked BCMA or GPRC5D expression showed significant improvement, underscoring the versatility of this treatment.
**Key Results from the Study:**
– 21 patients with advanced, heavily pre-treated R/R MM participated.
– Of the 12 patients who received the optimal dosage of 2.0×10⁶ CAR-T cells/kg, 86% showed clinical response, with 75% achieving complete remission.
– Importantly, patients with BCMA or GPRC5D-negative cancer cells also responded well to treatment.
– The treatment was well-tolerated, with manageable side effects, including 71% of patients experiencing mild to moderate cytokine release syndrome (CRS).
This trial, published in *The Lancet Haematology*, represents a major step forward in MM treatment. The dual-target approach addresses the limitations of BCMA-targeted therapies, offering new hope to patients who have exhausted conventional options.
Looking ahead, this innovative therapy could reshape the future of multiple myeloma treatment, offering a powerful new tool in the fight against this complex disease.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#CancerResearch #MultipleMyeloma #CAR_TTherapy #MedicalInnovation #OncologyBreakthrough
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese Medical Team Research: 64.6% Complete Remission Rate, Over Half of Patients Progression-Free for More Than Two Years! Targeted Therapy Combined with Chemotherapy is Safe and Effective as First-Line Treatment for Lymphoma
**Chinese Medical Team Research: 64.6% Complete Remission Rate, Over Half of Patients Progression-Free for More Than Two Years! Targeted Therapy Combined with Chemotherapy is Safe and Effective as First-Line Treatment for Lymphoma**

Lymphoma
Peripheral T-cell lymphoma (PTCL) is a group of highly aggressive and heterogeneous diseases with poor prognosis. The standard CHOP regimen (cyclophosphamide, doxorubicin, vincristine, and prednisone) is widely used as first-line treatment for PTCL. Previous studies have confirmed the safety and efficacy of combining various targeted drugs with CHOP (CHOPX) for PTCL treatment. However, there are multiple PTCL subtypes with different mutations, such as TP53, TET2, KMT2D, and CREBBP/EP300, and evidence is still lacking regarding the effectiveness of these combined treatment regimens in patients with specific genetic mutations.
Recently, a Chinese study published in *The Lancet Regional Health–Western Pacific* showed that selecting targeted therapies based on specific genetic mutations, combined with CHOP as a first-line treatment for PTCL, demonstrated good efficacy and safety. Compared to standard CHOP, CHOPX treatment achieved a higher complete remission rate and longer progression-free survival. This study was conducted by a Chinese medical team.
This was an open-label, multi-center, non-randomized, external-controlled phase 2 clinical trial (code-named GUIDANCE-03) conducted across seven medical centers in China. The trial compared the efficacy and safety of targeted drug combinations based on specific genetic mutations with CHOP (CHOPX) versus CHOP alone in newly diagnosed PTCL patients.
A total of 96 newly diagnosed PTCL patients (aged ≥18 years, median age 63 years) were enrolled in the study, with 48 patients in each group (CHOPX and CHOP). Genetic sequencing results showed that 93 patients (96.9%) carried genetic mutations. Patients in the CHOPX group received standard CHOP treatment during the first treatment cycle. Starting from the second cycle, targeted drugs were added based on the patient’s specific genetic mutations: decitabine (for TP53 mutations), azacitidine (for TET2/KMT2D mutations), chidamide (for CREBBP/EP300 mutations), and lenalidomide (for patients without these mutations), with a total of six treatment cycles. Patients in the CHOP group received six cycles of CHOP treatment.
The primary endpoint of the study was the complete remission rate (CRR) at the end of treatment. Secondary endpoints included overall response rate (ORR), progression-free survival (PFS), overall survival (OS), and safety. The analysis results showed:
– At the end of treatment, the CRR of the CHOPX group was superior to that of the CHOP group. Compared to the CHOP group, the CHOPX group saw an approximate 30% improvement in CRR (64.6% vs. 33.3%, OR 0.27, 95% CI 0.12-0.64; P=0.004), achieving the primary endpoint of the study.
– For secondary endpoints, the ORR of the CHOPX group was better than that of the CHOP group (66.7% vs. 52.1%).
– During a median follow-up period of 24.3 months, the median PFS of the CHOPX group was significantly longer than that of the CHOP group (25.5 months vs. 9.0 months; HR 0.57, 95% CI 0.34-0.98; P=0.041), with a 43% reduction in the risk of disease progression or death. The 2-year PFS rates for the two groups were 53.2% (95% CI 38.7-67.7) and 28.0% (95% CI 13.6-42.3), respectively.
In terms of OS, the median OS for the CHOPX group has not yet been reached, while the median OS for the CHOP group was 30.9 months. The CHOPX group showed a trend toward improved OS, but the current statistical results are not significant (HR 0.55, 95% CI 0.28-1.10; P=0.088). The 2-year OS rates for the two groups were 68.0% and 60.8%, respectively.
Regarding safety, neutropenia was the most common adverse event in both the CHOPX and CHOP groups (82% in the CHOPX group and 73% in the CHOP group). The most common grade 3-4 hematologic adverse event in both groups was neutropenia, and the most common grade 3-4 non-hematologic adverse event was infection. In the CHOPX group, 65% (31 patients) reported neutropenia, but no patients experienced prolonged neutropenia (>14 days) or required dose adjustments of the targeted drugs, and 10% (5 patients) experienced infections. In the CHOP group, these proportions were 52% (25 patients) and 4% (2 patients), respectively.
In conclusion, the study results indicate that different targeted drugs combined with CHOP demonstrate good efficacy and safety. These findings provide preliminary evidence supporting the use of CHOPX as a first-line treatment for PTCL patients with different genetic mutations, and suggest that biomarker-driven treatment strategies are feasible and worth further exploration in the future.

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#CancerResearch #LymphomaTreatment #PTCL #TargetedTherapy #Chemotherapy #MedicalBreakthrough #Oncology #ClinicalTrials #ChineseMedicine #CancerSurvivorship #HealthcareInnovation #PrecisionMedicine #CancerTreatment #MedicalResearch #CHOPTherapy #OncologyUpdates #CancerCare
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Overcoming Challenges and Embracing New Life! – Breakthrough Progress of Eque-cel in the Treatment of Refractory Multiple Myeloma
### Overcoming Challenges and Embracing New Life! – Breakthrough Progress of Eque-cel in the Treatment of Refractory Multiple Myeloma

Multiple Myeloma
In the treatment of multiple myeloma (MM), how do we find new breakthroughs for patients who have not achieved complete remission (CR) after multiple rounds of chemotherapy? Research by Chinese medical professors has provided an exciting answer: Eque-cel (BCMA CAR-T therapy).
**Patient Background:**
This 58-year-old female patient was initially admitted to the hospital due to numbness and pain in both lower limbs and was eventually diagnosed with multiple myeloma. Despite receiving various treatment regimens, including VRD and SVPD, the results were unsatisfactory, and complete remission was not achieved. Faced with refractory characteristics, the doctors decided to try a more innovative treatment plan—CAR-T cell therapy.
**Treatment Process:**
In September 2023, the patient began peripheral blood mononuclear cell collection, followed by bridging therapy, and in November 2023, she received the Eque-cel infusion. Remarkably, just one month later, the patient achieved hematologic complete remission (CR) with minimal residual disease (MRD) negativity. After six months of follow-up, the patient maintained this excellent therapeutic effect.
**Professor’s Insights:**
Chinese medical professors pointed out that the advent of Eque-cel has brought new hope to refractory MM patients. The drug demonstrated significant efficacy in the FUMANBA-1 study: the overall response rate was as high as 98.9%, with 82.4% achieving complete remission, and 97.8% of patients achieving MRD negativity. The 12-month sustained MRD negativity rate reached 81.7%, and the PFS rate was 85.5%.
This outstanding result proves the significant advantage of Eque-cel in improving the depth of remission for MM patients, bringing hope for long-term survival to many refractory patients.
**Future Outlook:**
As the application and research of Eque-cel continue, we look forward to it providing better treatment options and survival opportunities for more MM patients. This new treatment plan is bringing a ray of hope to this stubborn disease and providing valuable experience for clinical experts worldwide.
**Stay Tuned:**
We will continue to follow the latest developments and research progress of Eque-cel, looking forward to its greater role globally, bringing hope and blessings to more patients.

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp:+8613717959070
Email: doctor.huang@globecancer.com
#MultipleMyeloma #CARTTherapy #MedicalBreakthrough #EqueCel #CancerTreatment #MedicalResearch #Hematology #PatientCare #OncologyInnovation #HopeForPatients #BCMA #CART #MM #RRMM
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese Medical Team: Long Survival and Significant Benefits with BCMA CAR-T Treatment for RRMM: A 5-Year Outlook
**Chinese Medical Team: Long Survival and Significant Benefits with BCMA CAR-T Treatment for RRMM: A 5-Year Outlook**

RRMM
Chimeric antigen receptor (CAR) T-cell therapy is one of the most promising new treatments for relapsed/refractory multiple myeloma (RRMM), but reports on its long-term efficacy and safety are limited. As early as 2022, Professor Du Juan’s team from the Department of Hematology at Shanghai Changzheng Hospital published a Phase I/II study demonstrating that patients with poor physical status could also benefit from CAR-T therapy. Recently, the team updated their findings with a five-year long-term follow-up, focusing on factors affecting long-term clinical benefits. The results were published in *Clinical Cancer Research*. The following summary of the study’s content is provided by *Cancer Information* for readers’ benefit.
### Evidence for Long-Term Efficacy and Safety of BCMA CAR-T Cell Therapy
#### Patient Characteristics
The study included 49 RRMM patients who had all received at least three prior lines of therapy before undergoing BCMA CAR-T cell treatment. At enrollment, 20 patients (40.82%) had poor physical status (ECOG performance status of 3-4), 42.86% had high-risk cytogenetic features, and 63.27% had received four or more lines of treatment. At the time of infusion, 79.59% had progressive disease. Among the patients with poor physical status, 30% had extramedullary disease (EMD), 45% had high-risk cytogenetic features, 70% had received four or more lines of treatment, and 80% had progressive disease after their last line of treatment.
#### Efficacy Evaluation of BCMA CAR-T Cell Therapy HDS269B
After a median follow-up of 59.0 months, the study showed an overall response rate (ORR) of 77.55%. The ORR was similar across patients with different ECOG scores. The median progression-free survival (PFS) was 9.5 months, and the median overall survival (OS) was 20.0 months. The five-year PFS and OS rates were 21.3% and 31.4%, respectively. For patients with ECOG scores of 0-2, the median PFS was 11.0 months, compared to 4.0 months for those with scores of 3-4 (P=0.18). The median OS was 41.8 months for ECOG 0-2 patients and 10.5 months for ECOG 3-4 patients (P=0.015).
Patients who had previously undergone four or more lines of therapy had significantly shorter PFS and OS compared to those who had received fewer than four lines (PFS: P=0.012; OS: P=0.0049). Among the 11 patients with EMD at enrollment, the ORR was 64% for those with EMD and 82% for those without EMD. However, median PFS and OS were notably shorter in patients with EMD (PFS: 3.0 months vs. 10.5 months, P=0.06; OS: 5.0 months vs. 24.0 months, P=0.03).
#### MRD-Negative Status and CAR-T Cell Persistence Indicate Better Long-Term Survival
Minimal residual disease (MRD) negativity was significantly associated with longer PFS and OS. In this study, MRD data were available for 22 patients on day 28 post-infusion, with 14 patients (63.64%) achieving MRD negativity (10^-4). These patients experienced significantly longer PFS and OS compared to MRD-positive patients. Similar associations were observed with MRD status at 3 and 6 months post-infusion.
The expansion of CAR-T cells was also closely related to clinical outcomes. Patients who achieved partial response (PR) or better had higher CAR-T cell peak levels. Patients without disease progression five years post-infusion had significantly higher CAR-T cell expansion peaks than those with progression. Additionally, the duration of CAR-T cell persistence correlated with longer PFS and OS, with patients having CAR-T cells persisting for ≥6 months, ≥12 months, ≥24 months, and ≥36 months showing significantly better PFS and OS than those without detectable CAR-T cells.
#### Controlled Safety Profile of BCMA CAR-T Cell Therapy HDS269B
No new CAR-T cell-related toxicities were observed during long-term follow-up. All patients experienced at least one adverse event (AE), with the most common long-term (≥28 days post-infusion) grade ≥3 AEs being hematologic in nature. No second primary malignancies or delayed immune effector cell-associated neurotoxicity syndrome (ICANS) were observed.
This study also included survival analysis, classifying patients by PFS and OS. The results indicated that ECOG 0-2 status, fewer than four prior therapies, and CAR-T cell persistence for ≥6 months were independently associated with longer survival.
### The Potential of BCMA CAR-T Therapy and the Need for Future Optimization
Through a five-year long-term follow-up of 49 RRMM patients, this study systematically evaluated the efficacy and safety of BCMA CAR-T cell therapy HDS269B. The findings suggest that poor physical status is not a contraindication for CAR-T therapy, thus broadening the indications for this treatment. While the results are encouraging, the study has some limitations, including its open-label, single-arm design and small sample size, which, combined with the long follow-up period, could lead to some patient attrition. Furthermore, despite the lack of new severe toxicities, long-term safety requires continued observation.
Overall, this study underscores the importance of BCMA CAR-T cell therapy in the treatment of RRMM and provides a crucial basis for exploring and applying CAR-T immunotherapy in the frontline treatment of multiple myeloma.

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +86137 1795 9070
Email: doctor.huang@globecancer.com
#CAR_Therapy #BCMACART #MultipleMyeloma #CancerResearch #LongTermSurvival #MedicalInnovation #Hematology #CancerTreatment #Immunotherapy #OncologyAdvances
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Successful CAR-T Treatment for Advanced Gastric Cancer! Beneficial for Liver Cancer, Brain Tumors, Pancreatic Cancer, with a DCR of 96.1%
Successful CAR-T Treatment for Advanced Gastric Cancer! Beneficial for Liver Cancer, Brain Tumors, Pancreatic Cancer, with a DCR of 96.1%

Gastric Cancer
According to a report by People’s Daily on April 6, 2023, China has achieved another milestone in the CAR-T field. Recently, China Jiangxi Province successfully completed its first case of CAR-T cell therapy for solid tumors. This not only represents a significant breakthrough in new cancer treatment technologies in Jiangxi but also marks a major advancement in China’s CAR-T research and application.
China’s Jiangxi Achieves a Breakthrough in CAR-T Therapy for Solid Tumors
The patient, an advanced gastric cancer patient, had previously undergone chemotherapy combined with immune checkpoint inhibitor (ICI) treatment, but the disease was not well controlled. The tumor had widely metastasized to the abdominal cavity and multiple bones, accompanied by complications such as anorexia, abdominal distension, and severe recurrent reduction in blood cells, making the patient unable to tolerate chemotherapy.
With no other options, the patient received CAR-T cell reinfusion therapy on March 20, 2023, at the Oncology Center of The Second Affiliated Hospital of Nanchang University, with substantial support from the team at Peking University Cancer Hospital (Beijing Cancer Hospital). Fortunately, the patient has safely passed the peak period of cytokine release syndrome (CRS). Compared to before CAR-T treatment, tumor markers have decreased to normal levels, and the patient’s physical condition, mental state, and symptoms (such as abdominal distension and anorexia) have significantly improved. This marks a “zero breakthrough” for CAR-T cell therapy in solid tumors in Jiangxi Province of China, leaving a significant mark in the field of CAR-T research and application in China.
Peking University Cancer Hospital Showcases CT041 CAR-T at 2024 ASCO Conference with a Disease Control Rate of 96.1%
In addition to this fortunate case, the research team from Peking University Cancer Hospital showcased a domestically developed CAR-T product named “satri-cel” (development code CT041) at the recent 2024 American Society of Clinical Oncology (ASCO) conference. Satri-cel is a CAR-T cell therapy targeting Claudin18.2 (CLDN18.2) and is the only CLDN18.2 CAR-T cell therapy in the world approved for clinical trials (IND) in both China and the United States, targeting digestive tract tumors such as gastric cancer and pancreatic cancer.
The final results of the Phase 1 clinical trial (NCT03874897) were presented in an oral report at this conference. In patients with gastric cancer/gastroesophageal junction adenocarcinoma (GC/GEJ) treated with satri-cel monotherapy, the results showed a disease control rate (DCR) of 96.1% (49/51), an objective response rate (ORR) of 54.9% (28/51), a median overall survival (mOS) of 9.0 months, a median progression-free survival (mPFS) of 5.8 months, and a median duration of response (mDOR) of 6.4 months.
As early as May 2022, the team at Peking University Cancer Hospital had reported clinical cases of CT041 treating gastric cancer. A 57-year-old gastric cancer patient (Pt08), who had previously undergone three treatments, including PD-1 antibody, showed improvement after receiving CT041 CAR-T cell therapy. Visual inspection and CT scans indicated that the patient’s condition improved, with reduced umbilical tumor lesions, and the efficacy was still ongoing as of the date of the report.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#CART #CancerTreatment #GastricCancer #LiverCancer #BrainTumor #PancreaticCancer #MedicalBreakthrough #Oncology #Jiangxi #ChinaBiotech #CancerResearch #ASCO2024 #PekingUniversityCancerHospital #InnovativeTherapy #MedicalAdvancements #HealthNews
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Breakthrough in mRNA Cancer Vaccine! Chinese Medical Team Brings New Hope to Advanced Cancer Patients Resistant to Traditional Treatments
Breakthrough in mRNA Cancer Vaccine! Chinese Medical Team Brings New Hope to Advanced Cancer Patients Resistant to Traditional Treatments

Cancer Vaccine
The rise of mRNA vaccines during the COVID-19 pandemic has inspired their use in cancer treatment. Recently, Professor Shen Baiyong’s team at Ruijin Hospital published a groundbreaking study in *Cell Research*. They reported the first therapeutic effects of an mRNA cancer vaccine targeting the KRAS G12V mutation in solid tumors globally, offering new hope to advanced cancer patients resistant to traditional treatments.
#### Research Highlights
Two patients with the KRAS G12V mutation and HLA-A*11:01 positivity were treated with the KRAS G12V mRNA vaccine and PD-1 inhibitors.
– **First Case**: An 86-year-old woman with pancreatic cancer showed significant lesion reduction and improved quality of life after treatment.
– **Second Case**: A 69-year-old man with advanced lung cancer experienced partial tumor regression and no notable side effects after treatment.
#### Significance
This study highlights the efficacy of mRNA cancer vaccines in treating advanced solid tumors. The KRAS G12V mutation has strong binding affinity with HLA-A11:01, common in Asians, suggesting many could benefit from this therapy.
Professor Shen, who leads the Pancreatic Disease Treatment Center at Ruijin Hospital, focuses on comprehensive pancreatic cancer care and has initiated clinical trials on mRNA vaccines for advanced patients.
#### Clinical Trials
Ruijin Hospital is conducting trials on fixed single-target, multi-target personalized, and postoperative preventive mRNA vaccines, promising new hope for patients. For more information, please contact us.
This innovative research could bring new treatment options to more patients worldwide.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#CancerResearch #mRNAVaccine #MedicalBreakthrough #AdvancedCancer #InnovativeTreatment #Healthcare #Oncology #ChineseMedicalTeam #NewHope #CancerTreatment #CellResearch
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Several Things Cancer Patients Need to Know Before Seeking Medical Treatment in China
Top-ranking oncology hospitals and clinics:
https://www.medtourcn.com/introducing-chinas-top-10-leading-cancer-hospitals/
To reach out or visit the best oncology hospitals in China, contact us or send the electronic version or photo of your genetic testing report and diagnosis report.
email:doctor.huang@globecancer.com
WhatsApp:+8613717959070
Cost Comparison
Prices can vary depending on the institution and the specifics of the treatment but generally fall within these ranges:
-
Price Ranges : Oncology Treatments: $10,000 – $50,000
In general, medical procedures in China cost 30% to 70% less than in the United States or Western Europe. Using CAR-T as an Example: CAR-T therapy has always been notorious for its high prices. How expensive is it exactly?
Solely considering the drugs themselves, Novartis’ Kymriah is priced the highest, exceeding 3 million RMB, while other CAR-T therapies in the United States are priced at over 2 million RMB.
In contrast, three domestically produced Chinese CAR-T therapies are all priced at less than 1.3 million RMB, only one-third of Novartis’ Kymriah. Specifically, JW Therapeutics’ Carteyva (Relmacabtagene autoleucel) is priced at 1.29 million RMB, FOSUNKITE’s Yescarta (Axicabtagene ciloleucel) at 1.2 million RMB, and FUCASO (Equecabtagene ciloleucel) by IASO Biotech at 1.166 million RMB. Moreover, just a few days ago, Inaticabtagene Autoleucel, independently developed by Juventas Biotech, has been approved for market release, marking a qualitative breakthrough in the price of domestically produced Chinese CAR-T therapies, priced at 999,000 RMB per unit.
