Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
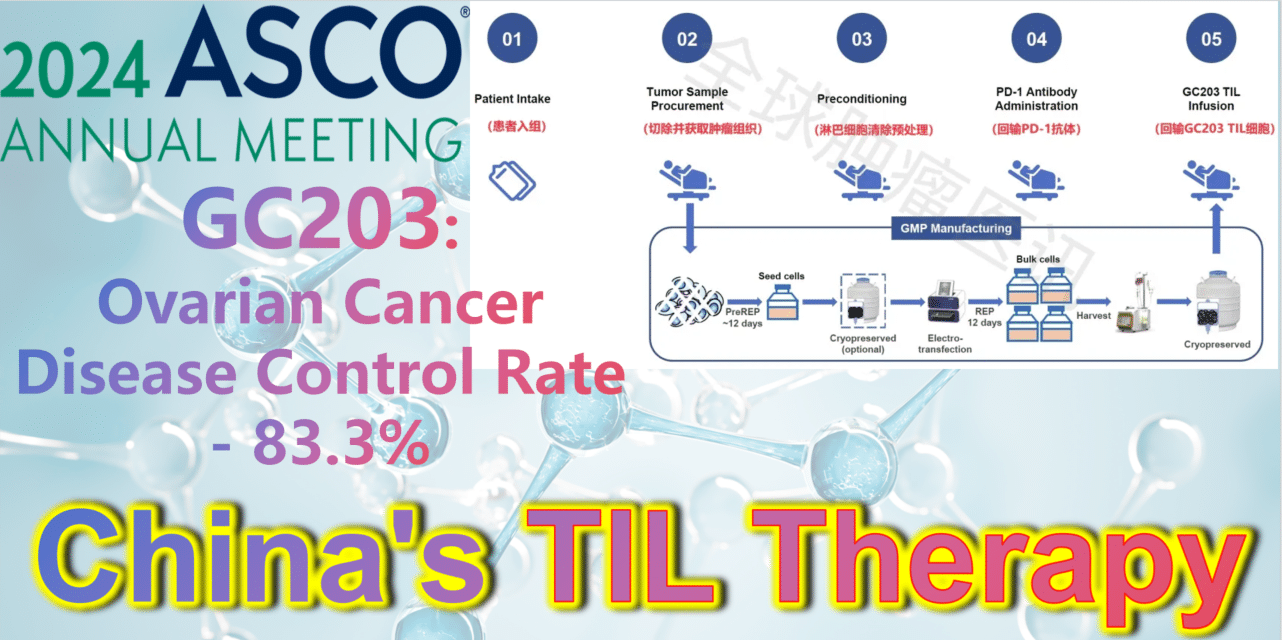
2024 ASCO China Voice: China’s TIL therapy – GC203: A Powerful Strike Against Ovarian Cancer with an 83.3% Disease Control Rate
2024 ASCO China Voice: China’s TIL therapy makes a grand debut, targeting ovarian cancer.
**GC203: A Powerful Strike Against Ovarian Cancer with an 83.3% Disease Control Rate**

TIL therapy
Ovarian cancer is a type of gynecologic tumor with a poor prognosis, with 70% of patients being diagnosed at a late stage. Unfortunately, effective treatment options for advanced ovarian cancer are quite limited, primarily relying on platinum-based chemotherapy. However, many ovarian cancer patients are not responsive to chemotherapy. Thus, there is an urgent need for new treatment options.
GC203 (mbIL-7-TIL) is a novel non-viral vector gene-modified TIL therapy utilizing membrane-bound IL-7. Developed by JunSai Biotech using the DeepTIL® cell expansion platform and NovaGMP® gene modification platform, it efficiently modifies T cells in a more economical way, enhancing the antitumor activity of TIL cells, activating internal immune cells, and avoiding systemic toxicity. It does not require lymphodepletion or combined IL-2 therapy post-infusion. A single patient is expected to save approximately 150,000 RMB in associated clinical costs, significantly improving the accessibility of TIL therapy and benefiting more cancer patients.
At the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, the latest clinical study results of GC203’s Phase 1 trial (NCT05468307) were announced. Between September 2021 and January 2024, 20 patients with recurrent ovarian cancer were enrolled, having undergone a median of 2.5 lines (range 1-9) of chemotherapy regimens (including PARP inhibitors, immune checkpoint inhibitors, etc.). After enrollment, patients first underwent tumor tissue resection, which was transported to GMP for a 22-26 day preparation period. The cryopreserved infusion products were then returned to the clinical center. Finally, patients received lymphocyte depletion pretreatment (including cyclophosphamide and hydroxychloroquine), a one-time PD-1 antibody infusion, and GC203 TIL cell reinfusion therapy.
After a median follow-up of 8.7 months (range, 2.9-18.8 months), results from 18 evaluable patients showed the following:
-
**Objective Response Rate (ORR):** The ORR in evaluable patients (n=18) was 33.3% (95% CI: 16.3%-56.3%). Among them, 22.2% (4 patients) achieved partial response (PR), and 11.1% (2 patients) achieved complete response (CR).
-
**Disease Control Rate (DCR):** The DCR in evaluable patients (n=18) was 83.3% (95% CI: 60.8%-94.2%).
-
**Median Progression-Free Survival (PFS):** The median PFS was 5.5 months (range, 1.0-14.1 months).
-
**Overall Survival (OS) Rate:** The 6-month OS rate was 75.6% (95% CI: 57.4%-99.6%); the 12-month OS rate was 68.8% (95% CI: 49.3%-95.9%).
-
**Adverse Reactions:** Most treatment-emergent adverse events (TEAEs) were grade 1 or 2, with common adverse reactions including elevated C-reactive protein levels (33%), fever (33%), and fatigue (11%), which could be alleviated or cured with symptomatic treatment. No other serious adverse reactions were observed.
In summary, for patients with recurrent or metastatic ovarian cancer with limited treatment options, GC203 TIL cell reinfusion therapy has shown good efficacy. Due to low-intensity pretreatment and no need for combined IL-2 therapy, its safety is significantly improved compared to traditional TIL therapy.
**How to Seek Help from TIL Therapy?**
The good news is that several TIL therapy clinical trials are currently recruiting in China, primarily targeting various solid tumors such as non-small cell lung cancer, melanoma, cholangiocarcinoma, esophageal squamous cell carcinoma, head and neck squamous cell carcinoma, breast cancer, ovarian cancer, cervical cancer, endometrial cancer, fallopian tube cancer, urothelial cancer, and renal cancer.
Patients seeking help from TIL therapy can submit their complete treatment history, recent pathology reports, imaging examination reports, and discharge summaries to Advanced Medicine in China.
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#OvarianCancer #TILTherapy #CancerTreatment #ASCO2024 #GC203 #Immunotherapy #MedicalResearch #Biotech #Oncology #ClinicalTrials #CancerInnovation #JunSaiBiotech #TIL #CancerBreakthrough #PatientCare #MedicalAdvancements
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Breakthrough Ultra CAR-T Therapy Shows High Disease Control Rate of 85.7% in Advanced Platinum-Resistant Ovarian Cancer

