Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

New Advances in Chinese CAR-T Cell Therapy: CD27 Armored BCMA CAR-T Shows Potential in Treating Relapsed Multiple Myeloma
**New Advances in Chinese CAR-T Cell Therapy: CD27 Armored BCMA CAR-T Shows Potential in Treating Relapsed Multiple Myeloma**

Multiple Myeloma
#BCMA #CD27#CARTCellTherapy #RRMM #MM #MultipleMyeloma #CART
Relapsed and refractory multiple myeloma (RRMM) is a challenging blood cancer that poses significant obstacles for patients. Although several BCMA (B-cell maturation antigen) targeting CAR-T cell therapies have been developed, achieving long-term remission and extended survival remains difficult. The recent introduction of CD27-armored BCMA CAR-T—CBG-002—by a Chinese medical team brings new hope for RRMM patients. Based on preclinical data, this therapy shows promising anti-myeloma activity, with Phase I clinical trial results further validating its efficacy and safety.
Research Highlights
CBG-002 is an innovative therapy that enhances traditional BCMA CAR-T by adding a CD27 costimulatory domain. The inclusion of CD27 improves CAR-T cell persistence and activity, contributing to stronger anti-cancer effects. Recently published Phase I research in Cancer Immunology Research indicates that this CD27-armored BCMA CAR-T therapy is not only well-tolerated but also shows notable efficacy in RRMM patients.
The study enrolled 11 RRMM patients, all of whom had undergone three or more prior treatments, with a median age of 54. The results showed that 81.8% of patients experienced only mild cytokine release syndrome (CRS) and no severe neurotoxicity. Even at a low dosage level (1-3×10^6 CAR-T cells/kg), CBG-002 achieved a high overall response rate (ORR) of 81.8%, with 45.5% of patients reaching stringent complete remission.
Future Outlook
The Phase I study of CBG-002 not only confirmed its efficacy but also demonstrated advantages in production time and cost. Compared to current products on the market, CBG-002 has a shortened production cycle of just 10 days, making it a timely and affordable treatment option for more patients. Amid global progress in CAR-T cell therapies, the innovation by this Chinese medical team positions it as a significant force in advancing cancer research worldwide.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#MultipleMyeloma #CancerResearch #Immunotherapy #ChinaBiotech #Oncology
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

What Kind of Multiple Myeloma Patients Are Suitable for CAR-T Therapy in China?
# What Kind of Multiple Myeloma Patients Are Suitable for CAR-T Therapy in China?

Multiple Myeloma
#MultipleMyeloma #CAR_Therapy #MM #CART #RRMM #Hematology
**Multiple Myeloma** (MM) is a malignant hematological disease that affects plasma cells. In recent years, various drugs and treatment methods, such as proteasome inhibitors, immunomodulatory drugs, and autologous stem cell transplantation, have significantly improved the prognosis of multiple myeloma patients. However, a considerable number of patients still experience relapses and refractory disease. For these patients, CAR-T cell therapy is emerging as a groundbreaking and effective treatment option.
China has made remarkable progress in CAR-T therapy technology in recent years, becoming a global leader in the field of hematological diseases. This has attracted patients worldwide to seek this advanced treatment. So, which multiple myeloma patients are suitable to receive CAR-T treatment in China?
## 1. **Indications: Preferred Choice for Drug-Resistant/Relapsed/Refractory Multiple Myeloma Patients**
CAR-T therapy is typically recommended for multiple myeloma patients who have developed resistance to standard treatments (such as proteasome inhibitors, immunomodulatory drugs, and monoclonal antibodies) and are in their third line of treatment or beyond. In China, CAR-T therapy has broader indications and better efficacy.
### Suitable Patient Types:
-
Relapsed or refractory multiple myeloma patients, typically those who have undergone at least second-line treatments.
-
Patients with high-risk genetic characteristics, such as certain mutations (e.g., 17p deletion, t(4;14) translocation).
-
Patients unable to tolerate traditional treatments, including chemotherapy, other targeted therapies, stem cell transplantation, or immunomodulatory treatments, due to poor tolerance or severe side effects.
-
Patients who are BCMA-positive.
-
Patients whose disease continues to progress despite other treatments, especially chemotherapy.
-
Patients who are ineligible for stem cell transplantation.
-
Patients with severe symptoms not responsive to conventional treatments, such as bone pain, anemia, hypercalcemia, and kidney damage.
-
High tumor burden patients—experts generally reduce the tumor burden first before administering CAR-T therapy, often through bridging or sequential treatments.
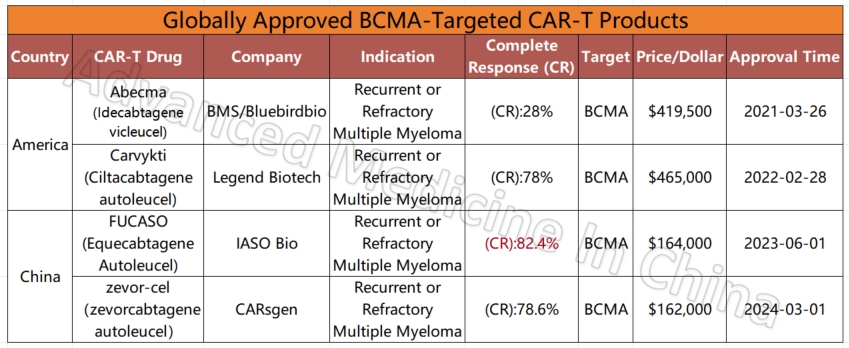
Among the four global CAR-T products targeting the BCMA marker in multiple myeloma, two Chinese products stand out for their efficacy and affordability. The most effective product currently is IASO Bio’s FUCASO (Equecabtagene Autoleucel), with a complete response (CR) rate of 82.4%.
## 2. **Patients in Good Physical Condition**
CAR-T therapy is essentially a powerful immunotherapy. Although it has remarkable efficacy, it also carries some risks of side effects, including cytokine release syndrome (CRS) and neurotoxic reactions. China’s CAR-T treatment system is highly developed, with established treatment plans and consensus. The country has extensive experience in managing CRS, neurotoxins, and side effects, keeping the risks very low. Moreover, the expert doctors at Advanced Medicine in China have substantial experience in managing these risks. Nonetheless, patients are typically required to have stable physical health to withstand the risks associated with the treatment.
– Overall good physical function; patients with heart, lung, liver, or kidney dysfunction may require a secondary evaluation by an expert team.
– Patients need to score 0-1 on the ECOG performance status scale, indicating that they are capable of daily activities and self-care.
## 3. **Patients with Financial and Time Support for CAR-T Therapy**
CAR-T therapy’s high cost and complex manufacturing process require patients and their families to have financial support. CAR-T therapy in China is significantly more affordable than in Western countries. While CAR-T treatments in the U.S. may cost $600,000 to $700,000, China’s approved CAR-T treatments cost around $100,000, about one-fifth to one-seventh of U.S. prices. Nevertheless, it remains a high-cost treatment, so patients need to be fully aware of the expenses involved.
China has invested heavily in CAR-T research in recent years. Many top hospitals and research institutions conduct related clinical trials. China accounts for over 50% of global CAR-T clinical trials. If a patient qualifies for a clinical trial, participating in it could be a more economical option, offering access to the latest CAR-T therapies, sometimes costing only tens of thousands of dollars or even free.
The CAR-T treatment process is relatively long. It involves collecting T cells, modifying them, re-injecting them, and close monitoring, requiring the patient to have enough time and patience to complete the entire process. The fastest known case took two weeks to complete all steps and discharge with complete response (CR), but generally, the process takes about four weeks or longer, depending on the patient’s condition.
For patients who can afford the treatment and have the time to complete it, CAR-T therapy in China is undoubtedly an attractive option.
## Conclusion
China’s leading position in CAR-T cell therapy technology and lower treatment costs make it a popular destination for multiple myeloma patients worldwide. It offers new hope for relapsed or refractory multiple myeloma patients. By working with experienced medical teams, patients can receive more personalized treatment plans and benefit from the rapid development of CAR-T therapy globally.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CancerTreatment #ChinaMedicalInnovation #Immunotherapy #MyelomaTreatment #AdvancedMedicine #RelapsedMyeloma #CancerResearch
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
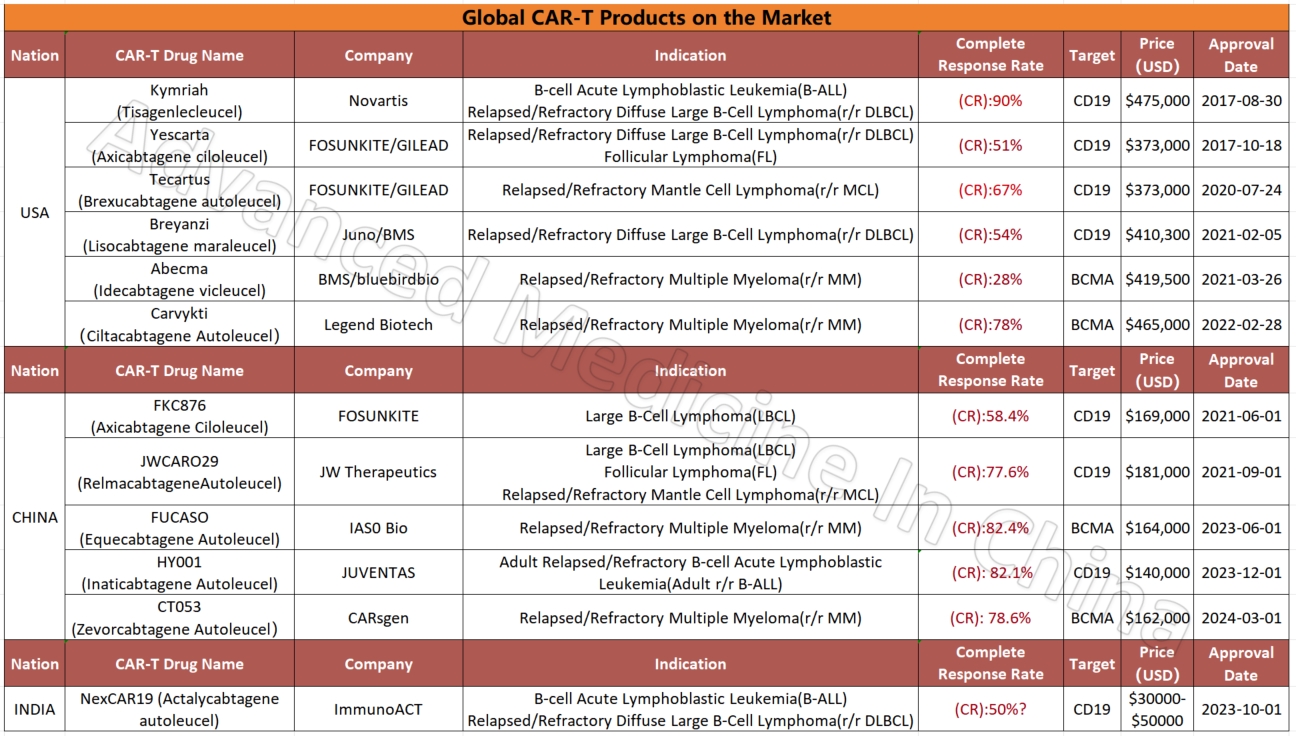
Global CAR-T Products on the Market and BCMA-Targeted CAR-T Products
Global CAR-T Products on the Market

CAR-T
Globally Approved BCMA-Targeted CAR-T Products
– Multiple Myeloma
Multiple Myeloma

 To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of Advanced Medicine in China for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

**Treatment for Multiple Myeloma: China’s BCMA×CD3 Bispecific Antibody Proposed for Breakthrough Therapy Designation**
**Treatment for Multiple Myeloma: China’s BCMA×CD3 Bispecific Antibody Proposed for Breakthrough Therapy Designation**

Multiple Myeloma
#MultipleMyeloma #BCMAxCD3 #BispecificAntibody #Immunotherapy #GR1803 #NMPA #RRMM
Recently, GR1803, an injectable solution developed by Genrix Bio, has been proposed for the “Breakthrough Therapy Designation” by the Center for Drug Evaluation (CDE) under China’s National Medical Products Administration (NMPA). This innovative drug targets relapsed and refractory multiple myeloma (RRMM), especially for patients who have undergone at least three lines of treatment, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody.
GR1803 is a novel bispecific antibody that simultaneously binds to BCMA and CD3 antigens. Its unique design allows for the effective activation of T-cells to attack tumor cells while minimizing non-specific T-cell activation, reducing potential side effects. In its Phase I clinical trial for RRMM, GR1803 demonstrated an overall objective response rate (ORR) of 85%, with an ORR of 100% in patients with extramedullary plasmacytoma (EMM).
The clinical performance of this product is remarkable, as evidenced by data presented at the 2024 European Hematology Association (EHA) annual meeting. As of January 2024, 40 trial participants have been enrolled, with an ORR of 85%. Notably, in the 180 ug/kg dose group, the median follow-up duration was 15 weeks, and 96% of the patients achieved a significant response.
What’s particularly noteworthy is the rapid response time; patients showed signs of improvement within a median of just 3 weeks. This suggests that GR1803 not only provides effective disease control in the short term but also continues to enhance patients’ conditions as treatment progresses.
The results from Genrix Bio’s research highlight that GR1803 offers new hope for RRMM patients, particularly for those who are unresponsive to conventional therapies. Its high response rate and favorable safety profile suggest that this drug could play a critical role in future cancer treatments.
The development of such groundbreaking drugs and the advancement of breakthrough therapies reflect China’s growing innovation in the global fight against cancer, offering new treatment options for patients worldwide.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#MultipleMyeloma #CancerTreatment #BreakthroughTherapy #Immunotherapy #BiotechInnovation #GenrixBio #BCMAxCD3 #CancerResearch #OncologyNews #PharmaInnovation
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Breaking Barriers: Equecabtagene Autoleucel Revives Hope for International RRMM Patients – Multiple Myeloma
Breaking Barriers: Equecabtagene Autoleucel Revives Hope for International RRMM Patients – Multiple Myeloma

Multiple Myeloma
Relapsed/refractory multiple myeloma (RRMM) presents a significant challenge worldwide, representing 13% of all blood cancers. Despite advances in treatment, many patients face poor outcomes and limited options. However, a groundbreaking CAR-T therapy, Equecabtagene Autoleucel, has recently demonstrated its life-changing potential in overcoming this medical impasse.
In February 2024, a 70-year-old patient from Kyrgyzstan, diagnosed with multiple myeloma 17 years ago, arrived at a Hospital of Chinese Xi’an seeking relief from severe pain and disease progression. The patient had undergone multiple treatments over the years, including chemotherapy and radiotherapy, but his condition continued to deteriorate with extensive bone destruction and kidney disease caused by the cancer.
Given the severity of his condition and the failure of previous treatments, he was selected for Equecabtagene Autoleucel therapy. After a series of preparatory treatments, the patient received CAR-T cell infusion in April 2024, and the results were remarkable. Within just one month, his myeloma cells were undetectable, and he achieved a complete response (CR) with no signs of minimal residual disease (MRD). The treatment was not only effective but also safe, with only mild side effects such as grade 1 cytokine release syndrome (CRS), which was easily managed.
This case exemplifies the transformative power of Equecabtagene Autoleucel, which has shown a 98.9% overall response rate (ORR) in clinical trials, with a CR rate of over 82%. It offers a beacon of hope for RRMM patients, particularly those who have exhausted conventional treatment options.
As one of China’s premier CAR-T therapies, Equecabtagene Autoleucel is breaking barriers, providing a new lease on life for patients globally. With its impressive safety profile and unparalleled efficacy, it is poised to play a pivotal role in extending survival and improving quality of life for those battling RRMM. This therapy is not only revolutionizing cancer treatment but also proving to be a lifeline for patients who previously faced limited options.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#MultipleMyeloma #CARTTherapy #CancerTreatment #MedicalInnovation #GlobalHealthcare #HopeForPatients #ChinaMedicalBreakthroughs #EquecabtageneAutoleucel #CAR_T #RRMM
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Redefining Hope for High-Risk RRMM Patients Worldwide: A Singaporean Patient`s Journey to China for Groundbreaking CAR-T Therapy
Redefining Hope for High-Risk RRMM Patients Worldwide: A Singaporean Patient`s Journey to China for Groundbreaking CAR-T Therapy

Multiple Myeloma
#MultipleMyeloma #CAR_Therapy #CancerTreatment #HRMM #MM #RRMM #CART
In the fight against multiple myeloma (MM), the last few decades have seen significant advancements, yet the disease remains notoriously difficult to cure, particularly in patients with relapsed/refractory multiple myeloma (RRMM). These patients face enormous challenges, as their options become increasingly limited after multiple lines of therapy have failed. However, hope has emerged in the form of BCMA CAR-T therapy, offering deep remission and long-term survival for those who had nearly lost hope.
One such case is a 58-year-old woman from Singapore, who after exhausting all available treatments in her home country, found new hope in China`s innovative CAR-T therapy. Diagnosed with MM in May 2021 following a month of severe back pain, she underwent a series of treatments including CD38 monoclonal antibodies, immunomodulatory drugs (IMiDs), proteasome inhibitors (PI), and XPO-1 inhibitors. Unfortunately, these therapies failed to halt the progression of her disease, which had become highly resistant to treatment.
In December 2023, she traveled to Beijing Chaoyang Hospital, Capital Medical University, where Professor Chen Wenming took charge of her case. The patient was diagnosed with high-risk MM (IgG-κ type) and admitted to the hospital on November 25, 2023, for CAR-T therapy.
#### The Treatment Journey: A Detailed Overview
Given the patient’s refractory nature and multiple prior treatments, Professor Chen devised a tailored treatment plan to improve her chances of survival and quality of life. In November 2023, her lymphocytes were collected to prepare the CAR-T cells. During this period, she received two cycles of D-PACE (dexamethasone, cisplatin, doxorubicin, cyclophosphamide, and etoposide) chemotherapy in Singapore to control the extramedullary plasmacytoma.
In February 2024, she returned to Beijing for further evaluation, where her condition was assessed as showing minimal response (MR). She was then administered a lymphodepletion regimen of fludarabine and cyclophosphamide on February 29. The following month, she received a transfusion of BCMA CAR-T cells.
Within five days post-transfusion, the patient developed a fever, which peaked at 39°C. Fortunately, her oxygen saturation and heart rate remained normal, and she was diagnosed with Grade 1 cytokine release syndrome (CRS). After symptomatic treatment, her blood counts recovered by day 15, and she was discharged in stable condition.
Two weeks post-CAR-T therapy, her response was evaluated as a very good partial response (VGPR), and by the two-month mark, her condition had improved to complete remission (CR) with minimal residual disease (MRD) negativity.
#### Insights from Leading Experts
Professor Wee Joo Chng, a specialist in high-risk MM, noted the aggressive nature of the patient’s disease, marked by genetic abnormalities like 1q21+ and t(4;14). Despite the use of multiple potent therapies, including KRd, XVd, and Isa-Pd, the patient’s disease continued to progress rapidly. The emergence of the del(17p) mutation further complicated her prognosis, indicating the need for a novel therapeutic approach.
The FUMANBA-1 study has highlighted the effectiveness of China`s indigenous CAR-T product, Equecabtagene Autoleucel, in achieving deep remission and prolonging survival in RRMM patients. This case demonstrated the therapy`s potential to overcome poor prognostic factors and extend the patient’s survival. Notably, the patient experienced only mild CRS and no immune effector cell-associated neurotoxicity syndrome (ICANS) or infections during treatment. At the two-month follow-up, the patient’s condition had improved to CR with MRD negativity, suggesting that Equecabtagene Autoleucel could be a game-changer for high-risk RRMM patients.
#### A New Frontier in CAR-T Therapy
RRMM patients with double-hit characteristics often experience early relapse and progression, leading to shortened survival times. Traditional therapies, including IMiDs, PIs, and monoclonal antibodies, have failed to overcome these poor prognostic factors, indicating the urgent need for novel treatments. Real-world studies have shown that CAR-T therapy offers comparable progression-free survival (PFS) and overall survival (OS) rates in RRMM patients, regardless of high-risk cytogenetic abnormalities.
The FUMANBA-1 study revealed impressive outcomes for Equecabtagene Autoleucel in RRMM patients, with an overall response rate (ORR) of 98.9% and an MRD negativity rate of 97.8% among CAR-T-naive patients. The CR rate was 82.4%, and 81.7% of patients maintained MRD negativity for over a year.
Globally, four CAR-T products are currently available, and a recent study presented at the 2024 European Society for Blood and Marrow Transplantation (EBMT) compared the short- and long-term efficacy of these therapies. The study’s matching-adjusted indirect comparison (MAIC) analysis revealed that Equecabtagene Autoleucel had a 12-month PFS rate of 94.2%, higher than the 75% observed with Ciltacabtagene autoleucel (CARTITUDE-1 study). Furthermore, the 12-month sustained MRD negativity rate for Equecabtagene Autoleucel was 100%, compared to 53.1% for Ciltacabtagene autoleucel.
These findings suggest that Equecabtagene Autoleucel, a Chinese-developed BCMA CAR-T therapy, offers superior long-term efficacy compared to its U.S. counterpart. As the global community celebrates the first anniversary of its approval, Equecabtagene Autoleucel continues to bring hope to RRMM patients worldwide, further solidifying China’s leading role in the field of cellular therapy.

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp:+8613717959070
Email: doctor.huang@globecancer.com
#ChinaMedicalInnovation #GlobalHealth #MMResearch #PatientCare #InnovativeMedicine #CellTherapy #MedicalBreakthrough
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Overcoming Challenges and Embracing New Life! – Breakthrough Progress of Eque-cel in the Treatment of Refractory Multiple Myeloma
### Overcoming Challenges and Embracing New Life! – Breakthrough Progress of Eque-cel in the Treatment of Refractory Multiple Myeloma

Multiple Myeloma
In the treatment of multiple myeloma (MM), how do we find new breakthroughs for patients who have not achieved complete remission (CR) after multiple rounds of chemotherapy? Research by Chinese medical professors has provided an exciting answer: Eque-cel (BCMA CAR-T therapy).
**Patient Background:**
This 58-year-old female patient was initially admitted to the hospital due to numbness and pain in both lower limbs and was eventually diagnosed with multiple myeloma. Despite receiving various treatment regimens, including VRD and SVPD, the results were unsatisfactory, and complete remission was not achieved. Faced with refractory characteristics, the doctors decided to try a more innovative treatment plan—CAR-T cell therapy.
**Treatment Process:**
In September 2023, the patient began peripheral blood mononuclear cell collection, followed by bridging therapy, and in November 2023, she received the Eque-cel infusion. Remarkably, just one month later, the patient achieved hematologic complete remission (CR) with minimal residual disease (MRD) negativity. After six months of follow-up, the patient maintained this excellent therapeutic effect.
**Professor’s Insights:**
Chinese medical professors pointed out that the advent of Eque-cel has brought new hope to refractory MM patients. The drug demonstrated significant efficacy in the FUMANBA-1 study: the overall response rate was as high as 98.9%, with 82.4% achieving complete remission, and 97.8% of patients achieving MRD negativity. The 12-month sustained MRD negativity rate reached 81.7%, and the PFS rate was 85.5%.
This outstanding result proves the significant advantage of Eque-cel in improving the depth of remission for MM patients, bringing hope for long-term survival to many refractory patients.
**Future Outlook:**
As the application and research of Eque-cel continue, we look forward to it providing better treatment options and survival opportunities for more MM patients. This new treatment plan is bringing a ray of hope to this stubborn disease and providing valuable experience for clinical experts worldwide.
**Stay Tuned:**
We will continue to follow the latest developments and research progress of Eque-cel, looking forward to its greater role globally, bringing hope and blessings to more patients.

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp:+8613717959070
Email: doctor.huang@globecancer.com
#MultipleMyeloma #CARTTherapy #MedicalBreakthrough #EqueCel #CancerTreatment #MedicalResearch #Hematology #PatientCare #OncologyInnovation #HopeForPatients #BCMA #CART #MM #RRMM
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese Medical Team: Long Survival and Significant Benefits with BCMA CAR-T Treatment for RRMM: A 5-Year Outlook
**Chinese Medical Team: Long Survival and Significant Benefits with BCMA CAR-T Treatment for RRMM: A 5-Year Outlook**

RRMM
Chimeric antigen receptor (CAR) T-cell therapy is one of the most promising new treatments for relapsed/refractory multiple myeloma (RRMM), but reports on its long-term efficacy and safety are limited. As early as 2022, Professor Du Juan’s team from the Department of Hematology at Shanghai Changzheng Hospital published a Phase I/II study demonstrating that patients with poor physical status could also benefit from CAR-T therapy. Recently, the team updated their findings with a five-year long-term follow-up, focusing on factors affecting long-term clinical benefits. The results were published in *Clinical Cancer Research*. The following summary of the study’s content is provided by *Cancer Information* for readers’ benefit.
### Evidence for Long-Term Efficacy and Safety of BCMA CAR-T Cell Therapy
#### Patient Characteristics
The study included 49 RRMM patients who had all received at least three prior lines of therapy before undergoing BCMA CAR-T cell treatment. At enrollment, 20 patients (40.82%) had poor physical status (ECOG performance status of 3-4), 42.86% had high-risk cytogenetic features, and 63.27% had received four or more lines of treatment. At the time of infusion, 79.59% had progressive disease. Among the patients with poor physical status, 30% had extramedullary disease (EMD), 45% had high-risk cytogenetic features, 70% had received four or more lines of treatment, and 80% had progressive disease after their last line of treatment.
#### Efficacy Evaluation of BCMA CAR-T Cell Therapy HDS269B
After a median follow-up of 59.0 months, the study showed an overall response rate (ORR) of 77.55%. The ORR was similar across patients with different ECOG scores. The median progression-free survival (PFS) was 9.5 months, and the median overall survival (OS) was 20.0 months. The five-year PFS and OS rates were 21.3% and 31.4%, respectively. For patients with ECOG scores of 0-2, the median PFS was 11.0 months, compared to 4.0 months for those with scores of 3-4 (P=0.18). The median OS was 41.8 months for ECOG 0-2 patients and 10.5 months for ECOG 3-4 patients (P=0.015).
Patients who had previously undergone four or more lines of therapy had significantly shorter PFS and OS compared to those who had received fewer than four lines (PFS: P=0.012; OS: P=0.0049). Among the 11 patients with EMD at enrollment, the ORR was 64% for those with EMD and 82% for those without EMD. However, median PFS and OS were notably shorter in patients with EMD (PFS: 3.0 months vs. 10.5 months, P=0.06; OS: 5.0 months vs. 24.0 months, P=0.03).
#### MRD-Negative Status and CAR-T Cell Persistence Indicate Better Long-Term Survival
Minimal residual disease (MRD) negativity was significantly associated with longer PFS and OS. In this study, MRD data were available for 22 patients on day 28 post-infusion, with 14 patients (63.64%) achieving MRD negativity (10^-4). These patients experienced significantly longer PFS and OS compared to MRD-positive patients. Similar associations were observed with MRD status at 3 and 6 months post-infusion.
The expansion of CAR-T cells was also closely related to clinical outcomes. Patients who achieved partial response (PR) or better had higher CAR-T cell peak levels. Patients without disease progression five years post-infusion had significantly higher CAR-T cell expansion peaks than those with progression. Additionally, the duration of CAR-T cell persistence correlated with longer PFS and OS, with patients having CAR-T cells persisting for ≥6 months, ≥12 months, ≥24 months, and ≥36 months showing significantly better PFS and OS than those without detectable CAR-T cells.
#### Controlled Safety Profile of BCMA CAR-T Cell Therapy HDS269B
No new CAR-T cell-related toxicities were observed during long-term follow-up. All patients experienced at least one adverse event (AE), with the most common long-term (≥28 days post-infusion) grade ≥3 AEs being hematologic in nature. No second primary malignancies or delayed immune effector cell-associated neurotoxicity syndrome (ICANS) were observed.
This study also included survival analysis, classifying patients by PFS and OS. The results indicated that ECOG 0-2 status, fewer than four prior therapies, and CAR-T cell persistence for ≥6 months were independently associated with longer survival.
### The Potential of BCMA CAR-T Therapy and the Need for Future Optimization
Through a five-year long-term follow-up of 49 RRMM patients, this study systematically evaluated the efficacy and safety of BCMA CAR-T cell therapy HDS269B. The findings suggest that poor physical status is not a contraindication for CAR-T therapy, thus broadening the indications for this treatment. While the results are encouraging, the study has some limitations, including its open-label, single-arm design and small sample size, which, combined with the long follow-up period, could lead to some patient attrition. Furthermore, despite the lack of new severe toxicities, long-term safety requires continued observation.
Overall, this study underscores the importance of BCMA CAR-T cell therapy in the treatment of RRMM and provides a crucial basis for exploring and applying CAR-T immunotherapy in the frontline treatment of multiple myeloma.

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +86137 1795 9070
Email: doctor.huang@globecancer.com
#CAR_Therapy #BCMACART #MultipleMyeloma #CancerResearch #LongTermSurvival #MedicalInnovation #Hematology #CancerTreatment #Immunotherapy #OncologyAdvances
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

2024 EHA | New Generation BCL-2 Inhibitor Sonrotoclax Expected to Be a New Treatment Option for RRMM
**2024 EHA | New Generation BCL-2 Inhibitor Sonrotoclax Expected to Be a New Treatment Option for RRMM**

RRMM
The 29th Annual Meeting of the European Hematology Association (EHA) was held from June 13-16, 2024, in Madrid, Spain. During this event, the results of the study on the new-generation BCL-2 inhibitor Sonrotoclax (BGB-11417) combined with dexamethasone for the treatment of relapsed/refractory multiple myeloma (RRMM) patients with t(11;14) were presented.
**Ib/II Phase Study of Sonrotoclax (BGB-11417) Combined with Dexamethasone for the Treatment of RRMM with t(11;14)**
**Study Background**
The B-cell lymphoma-2 (BCL-2) protein helps multiple myeloma (MM) tumor cells evade apoptosis and promote cell survival. Studies have found that MM cells with t(11;14) have significantly higher BCL-2 protein expression compared to other cells. BCL-2 inhibitors can block anti-apoptotic mechanisms and induce cell apoptosis. Currently, data have shown that BCL-2 inhibitors have significant anti-MM potential in RRMM patients with t(11;14) who have failed multiple lines of treatment. However, to date, no BCL-2 targeted therapy has been approved for MM treatment.
Sonrotoclax (BGB-11417) is a new-generation BCL-2 inhibitor. Preclinical studies have found that it has over ten times the BCL-2 inhibitory ability of the first-generation BCL-2 inhibitors, a shorter half-life, and no dose accumulation. BGB-11417-105 (NCT04973605) is an ongoing Ib/II phase trial aimed at evaluating the efficacy and safety of Sonrotoclax combined with dexamethasone ± carfilzomib/daratumumab/pomalidomide in treating RRMM patients with t(11;14). The latest data from this study were reported at this EHA meeting.
**Study Design**
The enrolled patients were all RRMM with t(11;14), who had failed at least three treatments, including proteasome inhibitors (PI), immunomodulatory drugs (IMIDs), and anti-CD38 monoclonal antibodies, and were refractory/relapsed to the most recent treatment. In the first part, dose escalation cohorts, patients took 80, 160, 320, or 640 mg of Sonrotoclax daily, combined with 40 mg of dexamethasone weekly until intolerance, disease progression, or death. The primary endpoints were safety/tolerability, determining the maximum tolerated dose (MTD)/maximum assessed dose (MAD), and recommending the dose for the expansion phase (RDFE). The second part, dose expansion, primarily evaluated the tolerability and antitumor activity of Sonrotoclax combined with dexamethasone ± carfilzomib/daratumumab/pomalidomide.
**Study Results**
The initial report at 2023 ASH showed that Sonrotoclax (640 mg) was well tolerated, with no dose-limiting toxicities (DLTs) observed in any patients, establishing 640 mg as the RDFE. The overall response rate (ORR) for this cohort was 70%, and the rate of very good partial response (≥VGPR) was 40%.
Updated in this report: as of March 25, 2024, 32 patients received the RDFE dose of 640 mg Sonrotoclax combined with dexamethasone (10 in the first part and 22 in the second part). The median follow-up time was 4.6 months (0.1-19 months).
The median age of patients was 69 years (48-80 years), with a median of 3 prior lines of treatment (1-12 lines), and 28.1% had high-risk cytogenetic abnormalities. All patients had been exposed to PI and IMiD treatments, and 72% had been exposed to anti-CD38 monoclonal antibody treatments. 56% were PI-refractory, 72% were IMiD-refractory, 56% were anti-CD38 monoclonal antibody-refractory, and 47% were triple-refractory.
**Safety**
No patients experienced DLTs. The most common treatment-emergent adverse events (TEAEs) were fatigue and insomnia (28% each), diarrhea (22%), constipation, and nausea (16% each). Only 4 patients (13%) experienced hematologic TEAEs (grade 3 thrombocytopenia, grade 1 and 3 platelet count decrease, and grade 3 neutropenia). Two patients died, both unrelated to treatment (one due to pancreatic cancer complications and one due to liver cancer and liver failure).
**Efficacy**
Among the 24 evaluable patients, the ORR was 75%, with a ≥VGPR rate of 50%. The complete response (≥CR) rate was 21% (CR, n=4; sCR, n=1), with two patients achieving minimal residual disease (MRD) negativity (10^-5). The median time to response was 0.7 months, and the median duration of response (DOR) was 8 months. As of the follow-up, the longest DOR was 18 months.
**Conclusion**
Sonrotoclax (640 mg) combined with dexamethasone was well tolerated in heavily pretreated RRMM patients with t(11;14), with low rates of hematologic toxicity and infection. It provided deep and durable responses: ORR was 75%, ≥VGPR rate was 50%, and ≥CR rate was 21%. This study will continue to explore Sonrotoclax in combination with other novel agents.
**Interpretation by Professor Lu Jin**
The t(11;14) translocation is a common genetic abnormality in MM patients, present in approximately 15%-20% of newly diagnosed MM cases. Before the era of novel drugs, many researchers believed that patients with t(11;14) had favorable treatment outcomes and were classified as a standard-risk group. However, recent studies have found that patients with t(11;14) are less sensitive to bortezomib regimens, and even with the VRd regimen, patients with t(11;14) have significantly lower deep response rates (≥VGPR) and PFS benefits compared to other standard-risk patients. This suggests that t(11;14) may be an influencing factor for poor efficacy of novel drugs, necessitating other therapies to improve prognosis.
Studies have found that tumor cells with t(11;14) often have high BCL-2 expression and high sensitivity to BCL-2 inhibitors. The phase III CANOVA study demonstrated that venetoclax combined with dexamethasone resulted in deeper response rates (ORR 62% vs. 35%, p<0.001; ≥VGPR rate 39% vs. 14%, p<0.001) and longer median PFS (9.1 months vs. 4.9 months, p=0.237) compared to pomalidomide combined with dexamethasone in treating RRMM patients with t(11;14), though without significant statistical difference. The failure to meet the primary endpoint might be due to more patients in the control group not reaching IMWG-defined disease progression and thus being treated with new regimens, resulting in censored data in the initial PFS analysis. In the latest post-hoc analysis, including new treatment as an event in PFS, the analysis showed a significant statistical difference in median PFS (9.4 months vs. 4.0 months, p=0.003).
Therefore, the benefits of BCL-2 inhibitors still warrant further exploration. Sonrotoclax is a second-generation highly selective and potent BCL-2 inhibitor. In preclinical trials, Sonrotoclax had an IC50 for BCL-2 protein over ten times lower than that of the first-generation BCL-2 inhibitors (0.014 nM vs. 0.2 nM). It also showed ≥2000 times selectivity over BCL-XL, BCL-W, MCL-1, and BCL2A1 and stronger cytotoxicity against MM cell lines. Additionally, Sonrotoclax has a shorter half-life (approximately 4.5 hours), allowing more flexible exploration of rapid dose escalation plans without the risk of off-target toxicity due to drug accumulation.
The Ib/II phase study reported at this EHA meeting demonstrated that Sonrotoclax combined with dexamethasone was well tolerated in RRMM patients previously treated with multiple lines of therapy. The 640 mg RDFE dose achieved a 75% ORR, ≥VGPR rate of 50%, and ≥CR rate of 21%. We look forward to the release of more results from Sonrotoclax combination therapies. Additionally, as the proportion of t(11;14) in systemic light chain amyloidosis and plasma cell leukemia is higher than in MM, the exploration of BCL-2 inhibitors in these plasma cell diseases is also ongoing.
**Professor Lu Jin**
Chief Physician, Professor, Ph.D. Supervisor
Peking University People’s Hospital, Peking University Institute of Hematology
Specializes in clinical and laboratory research on multiple myeloma, primary systemic amyloidosis, lymphoma, and cellular immunotherapy.
General Secretary and Standing Committee Member of the Hematology Physician Branch of the Chinese Medical Doctor Association
President of the Hematology Physician Branch of the Beijing Medical Doctor Association
Vice Chairman of the Multiple Myeloma Professional Committee and the Histiocyte Disease Professional Committee of the Chinese Medical Doctor Association
Vice President of the Hematology Branch of the Chinese Society of Geriatrics and Chairman of the Multiple Myeloma Academic Committee
Deputy Leader of the Plasma Cell Group of the Hematology Branch of the Chinese Medical Association
Deputy Leader of the Multiple Myeloma and Related Diseases Professional Group of the Hematology Professional Committee of the Chinese Women Physicians Association
Member of the Chinese and International Primary Systemic Amyloidosis Collaboration Group
Member of the International Myeloma Working Group and the Asia-Pacific Myeloma Working Group

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#Sonrotoclax #RRMM #BCL2Inhibitor #MultipleMyeloma #EHA29 #Hematology #NewTreatment #CancerResearch #2024EHA
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

What are the adverse reactions after CAR-T cell reinfusion?
 Interpretation of Hot Topics Conerning Mutiple Myeloma Patients:
Interpretation of Hot Topics Conerning Mutiple Myeloma Patients:
 “What are the adverse reactions after CAR-T cell reinfusion?”
“What are the adverse reactions after CAR-T cell reinfusion?”
Process Diagram for Myeloma Patients Receiving CAR-T Therapy
RRMM(Relapsed/Refractory Multiple Myeloma)
Unveiling how the “million-dollar anti-cancer shot” saves patients with recurrent and refractory bone tumors! Taking you through the entire process of CAR-T therapy.
 CAR-T细胞回输后有哪些不良反应?
CAR-T细胞回输后有哪些不良反应?
骨髓瘤患者接受CAR-T治疗的流程图
权威专家解读之骨髓瘤全程管理
Expert Introduction
Chief Phsician Professor Doctoral Supervisor
Peking University People’s Hospital,
Peking University Institute of Hematology
Director-General and Standing Committee of Hematolog Branch of Chinese Phsicians Association
Memer of the International Meloma oring Group Asia-Pacific Meloma oring Group
Associate Leader Plasma Ctolog Group Hematolog Branch Chinese Medical Association
Memer of the China and International Collaorative Group on Primar Sstemic Amloidosis
President of Hematologists Branch of Beiing Phsicians Association
Memer of International Research Group on Renal and Monoclonal lmmunogloulinopathies
Vice President of Hematolog Branch of Chinese Geriatrics Association and Chairman of Academic Commitee on Muliple Meloma
Associate Leader Multiple Meloma and Related Diseases Group Hematolog Committee Chinese Association of Female Phsicians

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#CART #Mutiplemyeloma #carttherapy #myeloma #leukemia #drug #RRMM #cancer #cancertreatment #tcell #cell #advancedmedical #AdvancedMedicineinChina
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

2024 EBMT : China’s First RRMM CAR-T Therapy Equecabtagene Autoleucel: Efficacy Unaffected by Patients’ Baseline sBCMA Plasma Levels
2024 EBMT : China’s First RRMM CAR-T Therapy Equecabtagene Autoleucel: Efficacy Unaffected by Patients’ Baseline sBCMA Plasma Levels

RRMM
In recent years, CAR-T cell therapy targeting BCMA has emerged as a groundbreaking treatment for multiple myeloma, offering new hope to patients. At the 50th European Society for Blood and Marrow Transplantation (EBMT) Annual Meeting, held from April 14-17, 2024, in Glasgow, the team led by Professor Qiu Lugui presented the latest subgroup analysis results from the FUMANBA-1 study (Abstract OS10-04) on China’s first BCMA-targeted CAR-T therapy, Iquilencel (CT103A).
BCMA (B-cell maturation antigen) is a promising therapeutic target for multiple myeloma (MM), with soluble BCMA (sBCMA) levels in the blood reflecting tumor burden. High sBCMA levels can interfere with the effectiveness of BCMA-targeted therapies, including CAR-T, by competing with cell-surface BCMA for binding, which can lead to reduced efficacy. In contrast, Iquilencel has been designed to minimize the impact of sBCMA on treatment outcomes through careful selection of its single-chain variable fragment (scFv).
The FUMANBA-1 phase II study (NCT05066646) in Chinese patients with relapsed/refractory multiple myeloma (RRMM) has demonstrated that Iquilencel can induce deep and durable responses, with a complete response (CR) rate of 82.4% and a 12-month progression-free survival (PFS) rate of 85.5%. This study aimed to explore whether baseline serum sBCMA levels affect clinical outcomes following Iquilencel infusion.
### Study Methods and Results
The study used enzyme-linked immunosorbent assay (ELISA) to measure serum sBCMA levels and digital droplet PCR (ddPCR) to monitor CAR transgene copy numbers in patients’ peripheral blood. Baseline serum sBCMA levels were classified into high (≥225.1 ng/mL) and low (<225.1 ng/mL) groups. Results showed that high sBCMA levels were significantly associated with high tumor burden, advanced R-ISS and DS stages, and high BCMA expression. However, there were no significant differences in CAR-T cell expansion, AUC (Area Under the Curve) during the first 28 days, or cell persistence between the high and low sBCMA groups.
Patients with high baseline sBCMA levels had overall response rates (ORR) and ≥CR rates of 100% and 80%, respectively, compared to 97.8% and 84% in the low sBCMA group. Analysis showed no significant correlation between baseline characteristics (including sBCMA levels) and CR/sCR achievement. Additionally, there were no significant differences in minimal residual disease (MRD) negativity rates, 18-month sustained MRD negativity rates, PFS, and overall survival (OS) between the two groups.
### Conclusion
The findings from the FUMANBA-1 study indicate that Iquilencel’s efficacy is not influenced by baseline sBCMA levels, making it a universally applicable and promising treatment option for RRMM patients. Its unique fast-dissociation and low-exhaustion properties, similar to those of healthy T-cell receptors, enable Iquilencel to remain effective and persistent in patients’ bodies regardless of sBCMA levels.
Professor Qiu Lugui from the Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Sciences, and Professor Li Chunrui from Tongji Hospital, Huazhong University of Science and Technology, noted, “sBCMA is an important biomarker of tumor burden in multiple myeloma and a key factor influencing prognosis. Accumulation of sBCMA can inhibit the function of BCMA CAR-T cells. However, our study shows that Iquilencel can overcome the challenges posed by high baseline sBCMA levels, providing significant and lasting responses for RRMM patients.”
These results underscore Iquilencel as an ideal treatment choice for RRMM, offering hope for more effective and long-lasting therapeutic outcomes.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#EBMT2024 #CAR_T #MultipleMyeloma #Iquilencel #EquecabtageneAutoleucel #sBCMA #CancerResearch #Immunotherapy #MedicalBreakthrough #Biopharmaceuticals
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

IMWG Releases 2024 RRMM CAR-T Guidelines: Equecabtagene Autoleucel Becomes China’s First Included CAR-T Therapy
###IMWG Releases 2024 RRMM CAR-T Guidelines: Equecabtagene Autoleucel Becomes China’s First Included CAR-T Therapy

IMWG
