Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
The Lancet | Chinese Medical Team: A New Breakthrough in the Treatment of Limited-stage Small Cell Lung Cancer!
The Lancet | Chinese Medical Team: A New Breakthrough in the Treatment of Limited-stage Small Cell Lung Cancer!
High-dose Accelerated Hyperfractionated Radiotherapy Helps Improve Treatment Efficacy

Lung Cancer
Small-cell lung cancer (SCLC) is a highly aggressive malignancy, accounting for 15% of all lung cancer cases. Among these, one-third are diagnosed with limited-stage SCLC (LS-SCLC), where the disease is confined to one side of the chest. For the past two decades, the standard treatment for LS-SCLC has been concurrent chemoradiotherapy, yet patients have seen little improvement in survival outcomes, with median survival ranging from 25-30 months.
A New Hope for LS-SCLC Patients
A recent study led by a team of Chinese researchers has shed light on a new treatment strategy. Published in The Lancet Respiratory Medicine, this multicenter, open-label, randomized phase III trial demonstrated that a high-dose, accelerated, hyperfractionated thoracic radiotherapy (54 Gy/30 fractions) combined with chemotherapy significantly improves overall survival (OS) and progression-free survival (PFS) for LS-SCLC patients. Importantly, this breakthrough treatment does not increase toxicity compared to the standard 45 Gy/30 fractions regimen.
Study Design and Methodology
The trial, conducted between 2017 and 2021, enrolled 224 patients across 16 hospitals in China. The patients, aged 18-70, had histologically or cytologically confirmed LS-SCLC and had either not yet received treatment or had undergone 1-2 cycles of chemotherapy. After chemotherapy, they were randomly assigned to receive either the high-dose (54 Gy/30 fractions) or standard-dose (45 Gy/30 fractions) thoracic radiotherapy, delivered twice daily over three weeks. Both groups also underwent four cycles of chemotherapy and, if responsive, prophylactic cranial irradiation (PCI) to prevent metastasis.
Improved Survival Outcomes
The study’s results were groundbreaking. Patients in the high-dose group showed a remarkable improvement in OS, with a median survival of 60.7 months, compared to 39.5 months in the standard-dose group. Additionally, the high-dose group saw a 45% reduction in the risk of death. Two-year survival rates also favored the high-dose group, with 76% of patients alive at two years, compared to 54% in the standard-dose group.
Progression-free survival was similarly improved, with the high-dose group experiencing a median PFS of 30.5 months, significantly longer than the 16.7 months observed in the standard-dose group. This translates to a 30% reduction in the risk of disease progression or death.
Safety and Tolerability
Despite the higher radiotherapy dose, the incidence of adverse effects between the two groups was comparable. The most common severe side effects were neutropenia, thrombocytopenia, and anemia, which occurred at similar rates in both groups. The occurrence of acute radiation-induced toxicity, such as esophagitis and radiation pneumonitis, also showed no significant differences. Importantly, there were no cases of severe late-stage toxicity, such as grade 3 esophageal stenosis or pulmonary fibrosis.
Clinical Implications and Future Directions
This study demonstrates that high-dose, accelerated hyperfractionated thoracic radiotherapy can significantly improve the prognosis of LS-SCLC patients without increasing treatment toxicity. It provides a new first-line treatment option that offers hope for longer survival. These findings may shift the clinical landscape, offering a viable alternative to the current standard of care.
As research continues to explore combining this high
A groundbreaking study led by a Chinese medical team has demonstrated that high-dose accelerated hyperfractionated chest radiotherapy (54 Gy/30 fractions) combined with chemotherapy significantly improves the overall survival (OS) of patients with limited-stage small-cell lung cancer (LS-SCLC). The research was published in The Lancet Respiratory Medicine on August 12, 2024. Compared to the standard dose of 45 Gy/30 fractions, the 54 Gy regimen increased OS without raising treatment toxicity, marking a potential shift in LS-SCLC treatment strategy.
Key Findings of the Study
LS-SCLC, a highly aggressive form of lung cancer, affects around 15% of all lung cancer patients, with one-third of them diagnosed at a limited stage. Traditionally, concurrent chemoradiotherapy (CRT) has been the preferred treatment option for LS-SCLC, yet the median survival rate has remained stagnant at around 25-30 months over the past two decades. The new study, however, highlights the efficacy of using higher doses of radiation to improve outcomes.
This phase III clinical trial was conducted across 16 hospitals in China between June 2017 and April 2021, involving 224 LS-SCLC patients. The trial compared two groups: a high-dose radiotherapy group (54 Gy/30 fractions twice daily) and a standard-dose group (45 Gy/30 fractions twice daily). Patients were treated with four cycles of chemotherapy and monitored through regular follow-ups. The results were remarkable.
Overall Survival: The high-dose group had a significantly extended median OS of 60.7 months compared to 39.5 months in the standard-dose group, reducing the risk of death by 45%.
Progression-Free Survival (PFS): The median PFS was also improved in the high-dose group, standing at 30.5 months compared to 16.7 months in the standard-dose group, with a 30% reduction in the risk of disease progression or death.
Safety: Despite the higher radiation dose, there was no increase in severe treatment-related side effects, such as neutropenia, thrombocytopenia, or radiation-induced pneumonia. Both groups exhibited similar rates of acute and long-term radiation toxicity.
Why Is This Study Important?
The study’s findings suggest that 54 Gy/30 fractions, delivered twice daily, may become a new standard of care for LS-SCLC patients. This approach significantly extends survival while maintaining a similar safety profile to the current standard dose. Moreover, with a two-year OS rate of 76% in the high-dose group, compared to 54% in the standard-dose group, the potential for long-term remission appears much greater.
A New Era in LS-SCLC Treatment?
While the current trial focused on radiotherapy and chemotherapy alone, future studies could explore combining this high-dose radiotherapy with immunotherapy. Recent breakthroughs, such as the ADRIATIC trial, have shown that immunotherapy following CRT can further improve survival outcomes for LS-SCLC patients. However, the safety of combining high-dose radiotherapy with immunotherapy needs further investigation due to potential risks like radiation-induced pneumonitis.
In conclusion, this study has paved the way for a new treatment option in LS-SCLC, offering hope for improved survival rates without additional toxicity. It marks a significant milestone in the battle against this aggressive form of lung cancer, providing a valuable alternative to the long-standing standard treatments.

 To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#LungCancerTreatment #SCLCTherapy #CancerResearch #MedicalBreakthrough #ChinaHealthcare #Oncology #LungCancerSurvival #Radiotherapy #CancerCare #ClinicalTrials
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
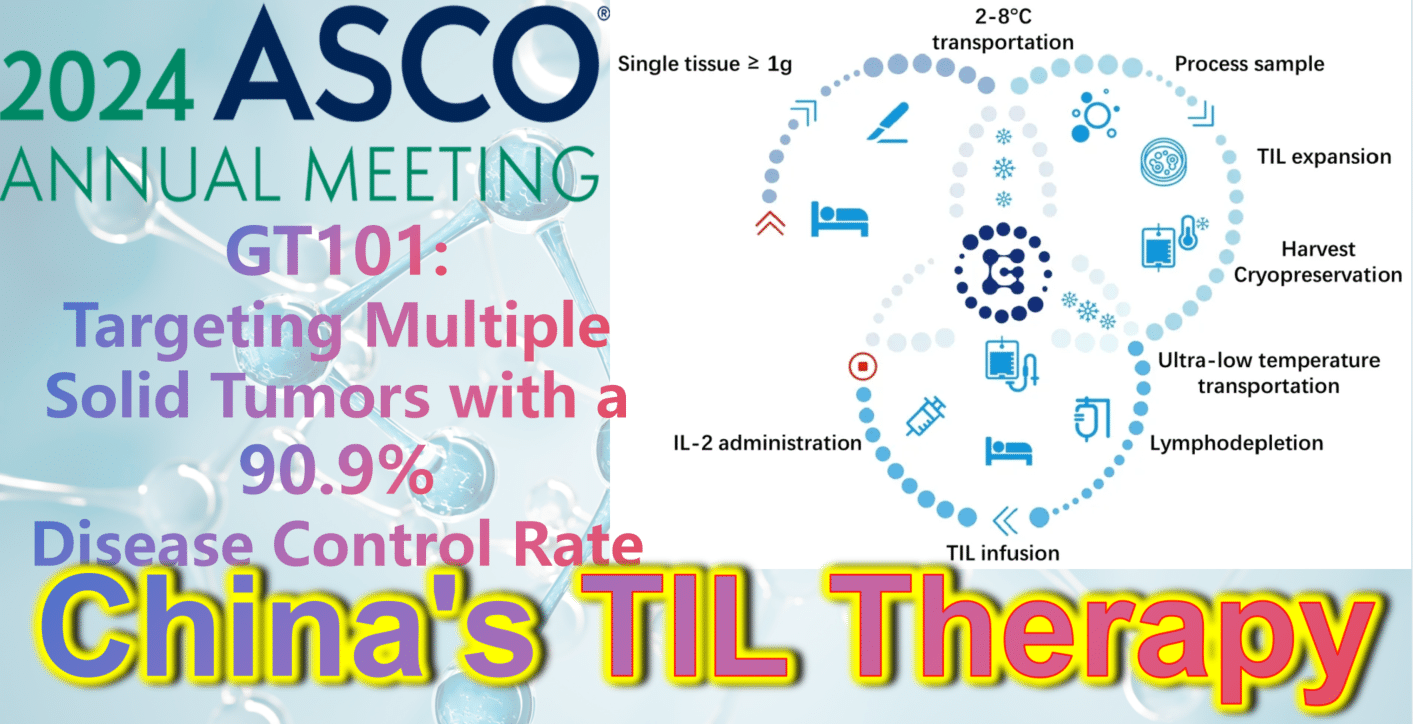
2024 ASCO China Highlights: China’s Indigenous TIL Therapy – GT101: Targeting Multiple Solid Tumors with a 90.9% Disease Control Rate
**2024 ASCO China Highlights: China’s Indigenous TIL Therapy Makes a Strong Debut, Targeting Small Cell Lung Cancer, Melanoma, Cervical Cancer, with a DCR Over 90%**
**GT101: Targeting Multiple Solid Tumors with a 90.9% Disease Control Rate**

TIL Therapy
GT101, independently developed by Gravel Biotech, is an autologous tumor-infiltrating lymphocyte (TIL) therapy. On April 22, 2022, its clinical trial implied consent was approved by the National Medical Products Administration (NMPA) (Acceptance No.: CXSL2200061). It is indicated for treating various solid tumors including non-small cell lung cancer, melanoma, and cervical cancer. Notably, GT101 is China’s first approved clinical TIL cell therapy and holds promise as the first cell therapy to conquer solid tumors!
Recently, at the 2024 ASCO Annual Meeting, results from the Phase 1 clinical trial of GT101 TIL therapy (NCT05430373) were announced. By November 10, 2023, a total of 14 patients with recurrent or metastatic solid tumors (including small cell lung cancer, melanoma, cervical cancer) had been enrolled, all with an ECOG performance status of 0 or 1 and having received a median of 2.6 prior lines of therapy. After enrollment, tumor tissue was obtained via appropriate surgical procedures for GT101 preparation, followed by approximately 30 days of TIL cell culture. Patients then underwent non-myeloablative lymphocyte depletion (cyclophosphamide + fludarabine), GT101 reinfusion therapy, and IL-2 (Interleukin-2) treatment.
1. **All enrolled patients (n=14):** The Objective Response Rate (ORR) was 35.7%. Among them, 28.6% (4 patients) achieved partial response (PR), 57.1% (8 patients) achieved stable disease (SD), and 7.1% (1 patient) achieved complete response (CR).
2. **In cervical cancer patients (n=11):** The Objective Response Rate (ORR) reached 45.5% (5/11). The Disease Control Rate (DCR) was as high as 90.9% (10/11), with 36.4% (4 patients) achieving partial response (PR) and 9.1% (1 patient) achieving complete response (CR). The median Progression-Free Survival (PFS) was 4.2 months. According to Kaplan-Meier statistics, the durations of complete response (CR) and progression-free survival (PFS) were 24 weeks and 36 weeks, respectively.
In conclusion, GT101 demonstrated promising clinical efficacy and manageable safety in combination with lymphocyte depletion and high-dose IL-2 treatment. Particularly in the treatment of cervical cancer, its objective response rate and duration of response are remarkable!
**How to Seek Help from TIL Therapy?**
The good news is that several TIL therapy clinical trials are currently recruiting in China, primarily targeting various solid tumors such as non-small cell lung cancer, melanoma, cholangiocarcinoma, esophageal squamous cell carcinoma, head and neck squamous cell carcinoma, breast cancer, ovarian cancer, cervical cancer, endometrial cancer, fallopian tube cancer, urothelial cancer, and renal cancer.
Patients seeking help from TIL therapy can submit their complete treatment history, recent pathology reports, imaging examination reports, and discharge summaries to Advanced Medicine in China
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#ASCO2024 #TILTherapy #GT101 #CancerResearch #SolidTumors #ClinicalTrials #LungCancer #Melanoma #CervicalCancer #Immunotherapy #ChinaBiotech #CancerTreatment
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese Innovation in Medicine: A New Milestone in Small Cell Lung Cancer Treatment!

 **Chinese Innovation in Medicine: A New Milestone in Small Cell Lung Cancer Treatment!**
**Chinese Innovation in Medicine: A New Milestone in Small Cell Lung Cancer Treatment!**


Small Cell Lung Cancer
In the field of medicine, each new drug represents a leap forward in disease treatment, bringing new light and hope to patients. On May 9, 2024, the highly anticipated novel PD-L1 inhibitor, Bevosubab Single Monoclonal Antibody, officially received approval from the National Medical Products Administration (#NMPA) of China! This marks a vibrant addition to the treatment options for patients with extensive-stage small cell lung cancer, adding a brilliant rainbow to the sky of treatment.
Bevosubab Single Monoclonal Antibody is a Class I innovative drug declared by the Chinese biopharmaceutical subsidiary, Sinda Pharmaceuticals, for first-line treatment of extensive-stage small cell lung cancer.
Bevosubab Single Monoclonal Antibody is extraordinary—it is a brand-new PD-L1 inhibitor that directly targets the immune escape mechanism of tumor cells, awakening the body’s immune system to eradicate cancer cells invisibly. Combined with Anlotinib Hydrochloride, Etoposide, and Carboplatin, it forms a powerful treatment alliance, bringing new hope to small cell lung cancer.
This is not just a treatment regimen, but a redemption. In the ETER701 study, this four-drug combination therapy has rewritten the history of first-line treatment for small cell lung cancer! Survival has nearly doubled, greatly increasing the chances of survival and bringing more hope to patients.
What’s even more exciting is that, while using the combination therapy, the occurrence of adverse reactions is extremely manageable. This means that while gaining a longer life span, patients do not have to overly worry about the potential negative impacts of treatment.
The approval of Bevosubab Single Monoclonal Antibody not only opens new treatment doors for patients with extensive-stage small cell lung cancer but also brings new directions for exploration in the medical field. We are full of anticipation and believe that this new drug will shine brightly in future clinical practice, bringing endless hope and vitality to more patients!
To assess whether the condition is suitable for new durg therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <#AdvancedMedicineinChina> for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
