Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China’s CAR-T Therapy Emerging in Glioma Treatment: Neurosurgery Team Wins Three International Awards
**China’s CAR-T Therapy Emerging in Glioma Treatment: Neurosurgery Team Wins Three International Awards**

Glioma
At the recently concluded 76th International Exhibition of Ideas, Inventions, and New Products (IENA) in Nuremberg, Germany, a Chinese neurosurgery research team achieved remarkable success. Their independently developed project, the “Fourth-Generation Dual-Target Optimized CAR-T Cell Therapy Platform for Neuro-oncology,” stood out among over 500 inventions from more than 30 countries and regions. The team was awarded the exhibition’s highest prize, a gold medal, and the Best Invention Award from the International Federation of Inventors’ Associations (IFIA).
### Glioma: A New Application Area for China’s CAR-T Cell Therapy
Glioma is a malignant brain tumor resistant to traditional treatments. Its complexity and invasion into functional brain areas significantly affect patients’ quality of life and survival rates. In 2022, glioma-related deaths in China reached 57,000. Against this backdrop, CAR-T cell therapy offers a breakthrough hope in neuro-oncology.
The award-winning project by the Chinese neurosurgery team overcame a series of critical challenges, including efficient therapeutic target screening, reversing the immunosuppressive microenvironment of solid tumors, and optimizing CAR-T enhancement sequences. Based on these advancements, they developed Tris-CAR-T dual-target cell therapy. This therapy innovatively targets tumor stem cells, effectively enhancing the proliferation and killing capacity of CAR-T cells. Preliminary clinical trial results indicate that this therapy is highly effective and safe. To date, it has received nine national invention patents and eight international patents, with four of these patents already successfully commercialized.
### Global Attention and China’s Rising Technological Expertise
During the exhibition, research institutions, industry experts, and media from around the world showed great interest in this technology, considering it a milestone marking China’s CAR-T cell therapy’s advancement to an internationally leading position in neuro-oncology. The Chinese neurosurgery research team was an early leader in optimizing and expanding CAR-T therapy, which now has the potential for application in other solid tumors, showing significant clinical value and development prospects.
The IENA, founded in 1948, is one of the world’s three major invention exhibitions, showcasing top global innovations. Its fair and impartial review process provides an authoritative platform for worldwide inventions. The IFIA, as a nonprofit international inventors’ organization, aims to promote inventors’ status and facilitate international cooperation, significantly influencing global intellectual property and technological innovation.
The achievements of the Chinese neurosurgery team mark China’s accelerated rise in CAR-T cell therapy. In the future, China’s CAR-T innovations are expected to bring new hope for neuro-oncology patients globally, positioning China as a leader in this field.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CAR-T #GliomaTreatment #Neurosurgery #ChinaInnovation #CancerResearch #Immunotherapy #InternationalAwards #TrisCAR-T #MedicalBreakthrough #NeuroOncology #IENA2024 #IFIA #TumorStemCells #ClinicalTrials #SolidTumor
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Exceptional Efficacy of CAR-T Therapy in China: Disease Control Rate for Advanced Liver Cancer Reaches 78% to 91.3%
### Exceptional Efficacy of CAR-T Therapy in China: Disease Control Rate for Advanced Liver Cancer Reaches 78% to 91.3%

Liver Cancer
#CARTTherapy #LiverCancer #SolidTumors
In recent years, China has made remarkable advances in CAR-T cell therapy, particularly demonstrating exceptional potential in liver cancer treatment. CAR-T cell therapy, or chimeric antigen receptor T-cell therapy, involves genetically engineering a patient’s T-cells to enable precise recognition and elimination of cancer cells. This innovative therapy has shown impressive outcomes in treating hematologic cancers and is now showing promising potential in fighting more complex solid tumors—especially liver cancer.
### **A Ray of Hope with CAR-T Therapy Amid High Incidence of Liver Cancer**
Liver cancer ranks among the top three cancers worldwide and poses a significant health threat. The high incidence of liver cancer, largely related to chronic hepatitis B infection, is particularly evident in China, which accounts for 42.4% of global liver cancer cases, with an annual increase of 368,000 new cases. For many liver cancer patients, especially those with advanced-stage disease, traditional treatments offer limited effectiveness. CAR-T therapy, however, provides new hope. Recent studies indicate that the disease control rate of CAR-T therapy for advanced liver cancer patients in China has reached an impressive 78% to 91.3%, showcasing its powerful treatment potential.
### **Patient Stories: CAR-T Therapy as a Turning Point in Life**
One inspiring case is that of Mr. Zhang from China. Long troubled by chronic hepatitis, he was diagnosed with advanced liver cancer in 2016. Despite undergoing liver resection, the cancer recurred with multiple intrahepatic metastases, spreading to retroperitoneal lymph nodes and forming a tumor thrombus in the inferior vena cava. After multiple rounds of embolization and ablation, the tumor remained uncontrolled. Given the limited efficacy of traditional treatments, doctors recommended CAR-T therapy. Following CAR-T treatment, Mr. Zhang’s tumors completely disappeared, and he has remained tumor-free for over seven years without severe side effects. This case highlights both the efficacy of CAR-T therapy and its potential to offer hope.
Another patient, Mr. Li, also benefitted from CAR-T therapy. When other treatments proved ineffective, doctors developed a liver cancer-specific CAR-T “special force” of four billion cells for him. Upon injection of these modified CAR-T cells, Mr. Li’s alpha-fetoprotein levels dropped rapidly, and the tumor gradually shrank. In subsequent treatments, Mr. Li’s tumor disappeared entirely, and his condition has remained stable long-term. This success story further underscores CAR-T therapy’s impressive results in treating liver cancer.
### **Future Outlook: Challenges and Opportunities for CAR-T Therapy in China**
While CAR-T therapy has achieved notable successes, challenges remain. Compared to hematologic cancers, the environment for treating solid tumors is more complex, and enhancing the efficacy of CAR-T cells in solid tumors requires ongoing optimization. However, the promising research and clinical results instill confidence in CAR-T therapy’s future in liver cancer treatment. Chinese researchers are actively working to refine CAR-T cell therapy, explore combination treatment strategies, and foster international collaboration to benefit more patients.
In the future, CAR-T therapy in China is expected to become a powerful tool in liver cancer treatment, offering new hope to thousands of patients. With ongoing technological advancements and deepening clinical practice, CAR-T therapy in China will continue to advance cancer immunotherapy and provide new possibilities for the health of liver cancer patients.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CancerTreatment #ChinaMedicalAdvances #CancerImmunotherapy #MedicalBreakthrough #CancerResearch #LiverCancerAwareness #AdvancedCancerTreatment #CancerSurvivors #BiotechInnovation #GeneTherapy #Oncology #MedicalInnovation
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China Overcomes Solid Tumor Challenge: CAR-T Technology IM96 Leads Innovation in Colorectal Cancer Treatment
**China Overcomes Solid Tumor Challenge: CAR-T Technology IM96 Leads Innovation in Colorectal Cancer Treatment**

Colorectal Cancer
#IM96 #CART #ColorectalCancer #CARTtherapy #ESMO #2024ASCO #SolidTumor
China has made remarkable progress in the field of innovative cancer treatment. In October 2024, a Chinese biotechnology company announced that IM96 CAR-T cell injection, developed in collaboration with Peking University Cancer Hospital, received clinical trial approval from the National Medical Products Administration (NMPA). This marks a significant milestone for China in applying CAR-T technology to solid tumor treatment. IM96 is the first CAR-T cell drug approved in China for the treatment of metastatic colorectal cancer and has become the sixth CAR-T product approved for this company.
### Breakthroughs in IM96 Technology and Clinical Trial Highlights
IM96 utilizes ActSep-CD3/CD28 sorting and activation magnetic beads from China’s National Biopharmaceutical Technology Innovation Center. This is a cutting-edge technology, marking the first time China’s GMP-grade micron magnetic beads have been used in a CAR-T cell drug clinical trial, laying an important foundation for improved efficacy and safety.
The clinical trial of IM96 is led by Peking University Cancer Hospital, with Professor Lin Shen, a renowned oncology expert, as the principal investigator. Its preclinical study data has gained international recognition and was selected for the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, attracting significant attention.
### Exceptional Efficacy and Safety
IM96 has demonstrated outstanding efficacy in preclinical studies. Data shows a Disease Control Rate (DCR) of 73.7%, an Objective Response Rate (ORR) of 50%, and a median Progression-Free Survival (mPFS) exceeding 10 months. These results indicate a strong potential for IM96 in controlling tumor progression in metastatic colorectal cancer.
Additionally, IM96’s safety has been well-validated in preclinical studies, with only 5.0% of patients experiencing neurotoxicity or severe Cytokine Release Syndrome (CRS), underscoring its advantage in managing side effects.
### Meeting the Urgent Need for Metastatic Colorectal Cancer Treatment
Colorectal cancer is the third most prevalent cancer worldwide, with 1.93 million new cases and over 930,000 deaths each year. In China, the incidence and mortality rates of this cancer are high, particularly among advanced-stage metastatic patients who have limited treatment options. As an innovative solid tumor CAR-T drug developed independently in China, IM96 will address the gap in CAR-T therapies for colorectal cancer, offering new hope for patients.
### International Recognition and Academic Achievements
IM96 has been selected for consecutive presentations at the 2023 European Society for Medical Oncology (ESMO) and the 2024 ASCO Annual Meeting. Its excellent preclinical data has garnered global attention in the oncology community. The research findings have been accepted for publication in the prestigious *Journal for ImmunoTherapy of Cancer*. These achievements not only highlight China’s strength in CAR-T innovation but also contribute new perspectives and strategies to global cancer treatment.
The approval of IM96 marks a new phase in China’s development of CAR-T therapies, especially for solid tumors, and is expected to provide more effective and safer treatment options for colorectal cancer patients worldwide. This breakthrough will further enhance China’s international standing in cancer treatment and inject new momentum into global advancements in anti-cancer technologies.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CancerTreatment #MedicalInnovation #ChinaBiotech #Oncology
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China`s Breakthrough TIL Therapy GT201 Shows Remarkable Results in Advanced Solid Tumor Treatment: 100% Disease Control Rate, 69% Tumor Reduction
**China`s Breakthrough TIL Therapy GT201 Shows Remarkable Results in Advanced Solid Tumor Treatment: 100% Disease Control Rate, 69% Tumor Reduction**

TIL Therapy
#TILTherapy #GT201 #SolidTumor #TIL #ASCO2024
Recent research results indicate that the next-generation gene-edited TIL (Tumor-Infiltrating Lymphocyte) therapy, GT201, independently developed by Chinese biotech company GRIT Biotechnology, has shown significant potential in combating various advanced solid tumors. Through a unique membrane-bound cytokine design and the advanced retroviral system StaViral, GT201 has successfully overcome the traditional TIL therapy`s excessive reliance on IL-2, significantly improving its efficacy and durability in cancer treatment.
At the 2024 ASCO conference, the research team presented the results of the Phase I clinical trial for GT201. A total of 7 patients with advanced solid tumors, including non-small cell lung cancer, malignant melanoma, cervical cancer, and ovarian cancer, were enrolled in the trial. These patients had previously undergone at least three different treatments and showed notable efficacy after receiving GT201.
The data revealed that, among the 7 evaluable patients, 3 (42.9%) achieved confirmed partial responses (PR), with one patient’s tumor shrinking by an impressive 69%. Additionally, 2 patients (28.6%) achieved stable disease (SD). Notably, all 3 patients in the non-small cell lung cancer subgroup achieved disease control (SD ≥ 24 weeks or PR), resulting in a 100% disease control rate. These findings not only highlight the efficacy of GT201 but also bring new hope to patients with advanced non-small cell lung cancer.
Another significant advantage of GT201 is its safety profile. Throughout the trial, the therapy demonstrated good tolerability with manageable side effects. This result suggests that GT201 has the potential to become a powerful and effective treatment option for patients with advanced solid tumors, especially in the field of non-small cell lung cancer, where its objective response rate and disease control durability are highly encouraging.
As the clinical trials of GT201 continue to progress, it is poised to lead the future of solid tumor treatment and further advance TIL therapies. We look forward to more patients benefiting from this groundbreaking treatment.
To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070(Https://wa.me/+8613717959070)
Email: doctor.huang@globecancer.com
#SolidTumorTreatment #NonSmallCellLungCancer #CancerImmunotherapy #GRITBiotechnology #CancerResearch #GeneEditing #CancerTreatmentInnovation
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Hope to Eradicate Lung Cancer, Colorectal Cancer, and Various Solid Tumors! China’s First Self-Replicating mRNA Vaccine JCXH-211 Successfully Launched~
Hope to Eradicate Lung Cancer, Colorectal Cancer, and Various Solid Tumors! China’s First Self-Replicating mRNA Vaccine JCXH-211 Successfully Launched~

mRNA Vaccine
Tumor immunotherapy, as an emerging method for diagnosing and treating tumors, has garnered widespread attention since its inception. Prior to the advent of tumor immunotherapy, the clinical treatment of solid tumors relied solely on traditional surgical removal, radiotherapy, and chemotherapy. Although these methods were effective, they came with significant drawbacks such as drug side effects and the risk of recurrence.
Since the 1990s, mRNA vaccines have been considered therapeutic vaccines. During the COVID-19 pandemic in 2020, mRNA vaccines were used in clinical treatments, saving countless lives. Today, as the advantages of mRNA vaccines are gradually being discovered, they are now applied in multiple fields.
The advent of China’s first self-replicating mRNA vaccine JCXH-211 undoubtedly marks a new breakthrough in the clinical diagnosis and treatment of various solid tumors in China.
**What is the mRNA Vaccine JCXH-211?**
JCXH-211 is the world’s first self-replicating RNA drug encoding human interleukin-12 (IL-12) to enter clinical trials. This srRNA vaccine delivers mRNA encoding IL-12 into the cytoplasm, continuously expressing IL-12 to enhance the body’s immune response against tumor cells.
In preclinical studies involving various mouse and PDX disease models, JCXH-211 has shown the ability to effectively kill tumor cells, eliminate distant tumors, and prevent tumor recurrence. Comprehensive GLP toxicology studies have also demonstrated good safety.
JCXH-211 has a broad range of indications and is expected to effectively treat multiple solid tumors. The anticipated prospects are as follows:
**1. Lung Cancer:**
As one of the leading causes of cancer death worldwide, lung cancer is often diagnosed at a late stage due to difficulties in early detection. JCXH-211, through continuous expression of IL-12 and activation of immune cells, is expected to effectively control the growth and spread of lung cancer cells.
**2. Colorectal Cancer:**
Current clinical treatments for colorectal cancer primarily involve surgery and radiotherapy/chemotherapy, which can remove visible tumors but have a high recurrence rate. Periodic administration of JCXH-211 may significantly reduce recurrence and improve patient survival.
**3. Soft Tissue/Chondrosarcoma:**
These tumors are highly invasive and cover a wide range of areas, often affecting surrounding soft tissues and progressing rapidly. Current treatments are limited to surgery and radiotherapy/chemotherapy, which, although somewhat effective, are not thorough, leading to high recurrence rates. JCXH-211 could become a new treatment method, enhancing the body’s immune response to combat these stubborn cancer cells.
**4. Skin Cancer:**
Clinical treatments for skin cancer mainly include surgery and radiotherapy. While most skin cancers have a good prognosis, the effects on intractable melanoma are poor. The expression of IL-12 can activate specific T cells, greatly aiding in the elimination of melanoma cells.
**High-Efficiency Activation and Safety of the mRNA Vaccine JCXH-211!**
mRNA vaccines not only encode antigens to induce specific immune responses against tumors but also possess inherent immunostimulatory properties. This dual mechanism allows JCXH-211 to activate the immune system more efficiently, enhancing the overall anti-tumor effect.
Moreover, the safety of mRNA vaccines is well-recognized. Since DNA is the genetic material of most species, including humans and viruses, and is transcribed into mRNA before being translated into proteins to perform functions in the body, injecting mRNA into the human body does not enter the cell nucleus, thereby eliminating the risk of genomic insertion mutations. Additionally, mRNA can be naturally degraded and excreted from the body, preventing accumulation. Thus, JCXH-211 presents lower potential risks and is safer.
Currently, clinical trials are progressing steadily. With the rapid advancement of tumor immunotherapy, JCXH-211 is expected to become a standard treatment for various solid tumors, offering hope to many cancer patients.

 To assess whether the condition is suitable for cancer vaccine or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for cancer vaccine or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#LungCancer #ColorectalCancer #SolidTumors #mRNAVaccine #JCXH211 #CancerTreatment #TumorImmunotherapy #MedicalBreakthrough #Biopharma #CancerResearch #HealthcareInnovation #CancerHope #MedicalScience #HealthTech #ClinicalTrials #CancerSurvivor #CancerAwareness #InnovativeMedicine #CancerVaccine #Vaccine #mRNA #JCXH
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

🔬✨ Overall Response Rate 50%! Chinese Research Entity’s Solid Tumor CAR-T Hits Colorectal Cancer
🔬✨ Overall Response Rate 50%! Chinese Research Entity’s Solid Tumor CAR-T Hits Colorectal Cancer

Colorectal Cancer
#Solidtumor
In the journey to conquer cancer, a significant breakthrough is stirring! Shanghai Sundise Biotech has successfully developed the first candidate product GCC19CART, based on its independently developed CoupledCAR® platform technology. This innovative autologous #CART therapy product is specifically designed to target solid tumors of recurrent/refractory metastatic colorectal cancer (R/R mCRC). Let’s delve into this milestone achievement together!
#GCC19CART
On April 19, 2022, Shanghai Sundise Biotech announced that its solid tumor CAR-T product GCC19CART had been granted Fast Track designation by the U.S. Food and Drug Administration (#FDA). This news implies that the development of GCC19CART will be accelerated, bringing hope and new treatment options to more colorectal cancer patients.
#NCCN
So, what is recurrent/refractory colorectal cancer? According to the definition by the National Comprehensive Cancer Network (NCCN), it refers to patients who have undergone multiple rounds of conventional treatment without success or have developed metastases, facing extremely limited treatment options. Current statistics show that the survival rate and objective response rate of such patients are extremely low, urgently requiring more effective treatment methods.
#colorectalcancer
GCC19CART has demonstrated significant clinical activity by targeting and eliminating cancer cells expressing the colorectal cancer tumor marker GCC. According to publicly available data, GCC is widely expressed in colorectal cancer and is highly expressed in metastatic colorectal cancer cells, making it an ideal target for treatment. Preliminary clinical trial results indicate that GCC19CART exhibits significant objective response rates and acceptable safety profiles in refractory metastatic colorectal cancer, bringing new hope to patients.
#ORR 50%
A multicenter Phase I dose-escalation clinical trial conducted in China showed that the objective response rate in the medium-dose group (2×106 CAR-T/kg) reached an encouraging 50%. This suggests that GCC19CART may become an important choice for treating recurrent/refractory colorectal cancer, bringing longer survival and better quality of life to patients.
#Hope
Colorectal cancer is a disease that seriously threatens human health, and the successful development and clinical trial results of GCC19CART bring us new hope. Let’s look forward together to the early clinical application of this innovative treatment method, bringing light to more patients in need of help!
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: 137 1795 9070
Email: doctor.huang@globecancer.com
💪💊 #ColorectalCancer #CARTtherapy #MedicalInnovation
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

No Tumor Recurrence for Nearly 3 Years, China’s National Research #TIL Therapy #GC101 Targets Various Solid Tumors!
 No Tumor Recurrence for Nearly 3 Years, China’s National Research #TIL Therapy #GC101 Targets Various Solid Tumors!
No Tumor Recurrence for Nearly 3 Years, China’s National Research #TIL Therapy #GC101 Targets Various Solid Tumors!

TIL Therapy
 In recent years, immunotherapy has emerged as a pivotal direction in the future of cancer treatment. Tumor-infiltrating lymphocytes (#TILs) are hailed as the “natural enemies” of cancer cells. TIL therapy developed based on patients’ own tumor-infiltrating lymphocytes boasts advantages such as rich tumor-specific targets, excellent tumor homing capabilities, strong infiltration abilities, and a high safety profile.
In recent years, immunotherapy has emerged as a pivotal direction in the future of cancer treatment. Tumor-infiltrating lymphocytes (#TILs) are hailed as the “natural enemies” of cancer cells. TIL therapy developed based on patients’ own tumor-infiltrating lymphocytes boasts advantages such as rich tumor-specific targets, excellent tumor homing capabilities, strong infiltration abilities, and a high safety profile.
 On February 16, 2024, #Iovance Biotherapeutics announced the #FDA approval of Lifileucel (LN-144) for the treatment of advanced melanoma progressing after PD-1 antibody therapy, marketed as #AMTAGVI™. This marks the global first approval of a TIL therapy and the first approval worldwide for T-cell therapy for solid tumors, marking a significant milestone! #LN144
On February 16, 2024, #Iovance Biotherapeutics announced the #FDA approval of Lifileucel (LN-144) for the treatment of advanced melanoma progressing after PD-1 antibody therapy, marketed as #AMTAGVI™. This marks the global first approval of a TIL therapy and the first approval worldwide for T-cell therapy for solid tumors, marking a significant milestone! #LN144
 On the domestic front in China, several companies have announced good news regarding the clinical approval of TIL pipelines. Overall, the enthusiasm for TIL therapies is on the rise and increasingly intense. Today, let me introduce to you the first independently developed TIL cell therapy #GC101 from Juncell Therapeutics.
On the domestic front in China, several companies have announced good news regarding the clinical approval of TIL pipelines. Overall, the enthusiasm for TIL therapies is on the rise and increasingly intense. Today, let me introduce to you the first independently developed TIL cell therapy #GC101 from Juncell Therapeutics.
 With no tumor recurrence for nearly 3 years, GC01TIL is the first autologous natural tumor-infiltrating lymphocyte injection developed by Juncell Therapeutics, which officially obtained clinical trial implicit approval from the National Medical Products Administration (#NMPA) on April 24, 2022.
With no tumor recurrence for nearly 3 years, GC01TIL is the first autologous natural tumor-infiltrating lymphocyte injection developed by Juncell Therapeutics, which officially obtained clinical trial implicit approval from the National Medical Products Administration (#NMPA) on April 24, 2022.
 Distinguished from Iovance’s approved AMTAGVI™ (#Lifileucel), innovative GC101TIL therapy requires patients to be treated in ordinary wards without the need for high-intensity pre-conditioning before TIL cell infusion or any IL-2 dosage post-infusion. This simplified clinical protocol ensures effective TIL cell proliferation within patients, greatly avoiding the risks associated with AMTAGVI™’s black box warnings (such as treatment-related deaths, persistent severe cytopenia, severe infections, cardiopulmonary and renal function damage), substantially improving the safety, convenience, and accessibility of TIL therapy.
Distinguished from Iovance’s approved AMTAGVI™ (#Lifileucel), innovative GC101TIL therapy requires patients to be treated in ordinary wards without the need for high-intensity pre-conditioning before TIL cell infusion or any IL-2 dosage post-infusion. This simplified clinical protocol ensures effective TIL cell proliferation within patients, greatly avoiding the risks associated with AMTAGVI™’s black box warnings (such as treatment-related deaths, persistent severe cytopenia, severe infections, cardiopulmonary and renal function damage), substantially improving the safety, convenience, and accessibility of TIL therapy.
 Currently, according to the latest revelations from Juncell Therapeutics, the Phase I clinical trial of Juncell Therapeutics’ autologous natural TIL cell injection GC101 is underway in eight authoritative tertiary hospitals in China, achieving significant efficacy.
Currently, according to the latest revelations from Juncell Therapeutics, the Phase I clinical trial of Juncell Therapeutics’ autologous natural TIL cell injection GC101 is underway in eight authoritative tertiary hospitals in China, achieving significant efficacy.
 GC101, targeting various types of advanced solid tumors including malignant melanoma, cervical cancer, and lung cancer, has demonstrated an objective response rate of over 35%. Four patients have achieved complete tumor remission (#CR), with the longest no-recurrence survival period approaching 3 years, without experiencing any severe adverse reactions related to treatment, significantly improving the safety, applicability, and accessibility of #TILtherapy. Let us look forward to more surprises that GC101 will bring in the future!
GC101, targeting various types of advanced solid tumors including malignant melanoma, cervical cancer, and lung cancer, has demonstrated an objective response rate of over 35%. Four patients have achieved complete tumor remission (#CR), with the longest no-recurrence survival period approaching 3 years, without experiencing any severe adverse reactions related to treatment, significantly improving the safety, applicability, and accessibility of #TILtherapy. Let us look forward to more surprises that GC101 will bring in the future!
 Currently, #Juncell Therapeutics’ GC101 clinical trial for the treatment of advanced solid tumors is actively recruiting :
Currently, #Juncell Therapeutics’ GC101 clinical trial for the treatment of advanced solid tumors is actively recruiting :
FOR FREE
 Key Inclusion Criteria:
Key Inclusion Criteria:
1. Aged 18-75 years, regardless of gender;
2. Definitive diagnosis of malignant solid tumors (including but not limited to #melanoma, #lungcancer, #cervicalcancer, #esophagealsquamouscellcarcinoma, #headandnecksquamouscellcarcinoma, #endometrialcancer);
3. Standard treatment failure or lack of effective treatment options;
4. At least 2 lesions, with the physical condition supporting minimally invasive surgical sampling.
 This clinical trial is free of charge for participants. If you wish to participate in this clinical trial, you need to submit your treatment history, recent imaging and blood test reports, infectious disease reports, and discharge summaries to <@Advanced Medicine in China for preliminary assessment.
This clinical trial is free of charge for participants. If you wish to participate in this clinical trial, you need to submit your treatment history, recent imaging and blood test reports, infectious disease reports, and discharge summaries to <@Advanced Medicine in China for preliminary assessment.
 Email: doctor.huang@globecancer.com,
Email: doctor.huang@globecancer.com,
 WhatsApp: +8613717959070
WhatsApp: +8613717959070
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese Medicine Team Achieves Global Milestone: First Successful Treatment of Pancreatic Cancer Patient with Innovative Dual-Targeted CAR-T Cell Therapy (KD-496)!
🎉 Chinese Medicine Team Achieves Global Milestone: First Successful Treatment of Pancreatic Cancer Patient with Innovative Dual-Targeted CAR-T Cell Therapy (KD-496)!

Dual-Targeted CAR-T
Chinese Medicine
KD-496
CAR-T
NKG2DL+ /CLDN18.2+ solid tumors
Hope
WhatsApp+8613717959070
doctor.huang@globecancer.com
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
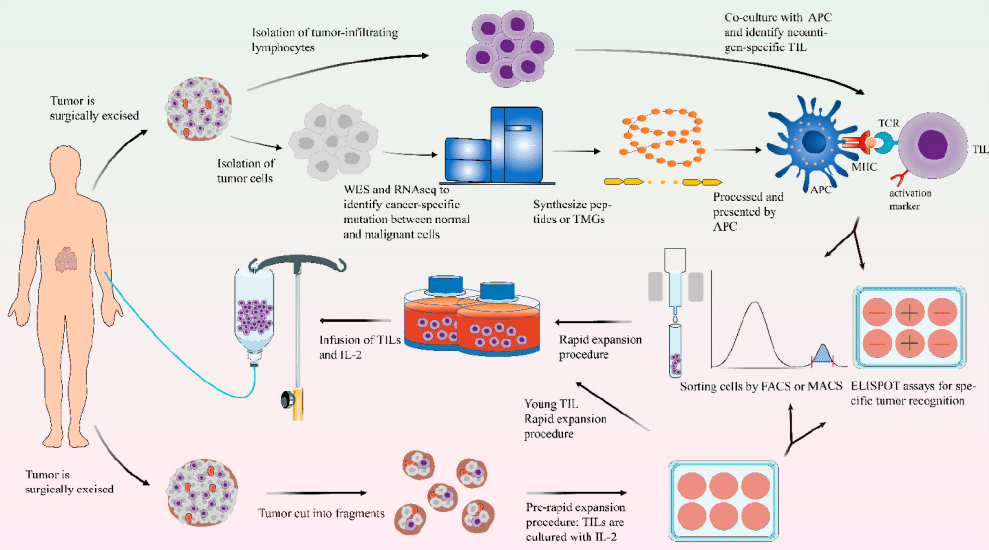
Unlocking the Potential: Understanding TIL Therapy for Solid Tumors
🔍 Unlocking the Potential: Understanding TIL Therapy for Solid Tumors🧬
💡What is TIL therapy?🧬
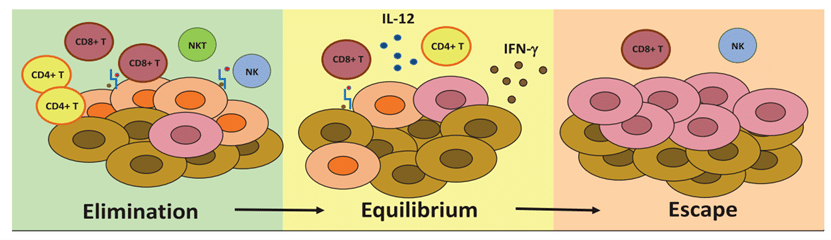
Cancer Development
Understanding Cancer Development: Immune Surveillance, Immune Equilibrium, and Immune Escape ���

TIL Therapy
🔬 The main routine steps of TIL therapy include:
💪Characteristics of TIL therapy:
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Breakthrough! Chinese Novel CAR-T Therapy Kills Tumors and Prevents Relapse!
🌟✨ **Breakthrough! Chinese Novel CAR-T Therapy Kills Tumors and Prevents Relapse!** ✨🌟

CAR-T Therapy
🔬 On January 2, 2024, a groundbreaking clinical study from China was published in *Nature*. This study, employing engineering design, enables CAR-T cells to secrete Interleukin-10 (IL-10), thereby enhancing metabolism within the tumor microenvironment. The modified IL-10 CAR-T cells increase oxidative phosphorylation in a mitochondrial acetoacetate carrier-dependent manner, resulting in complete regression of solid tumors and metastatic cancers, including colon cancer, breast cancer, melanoma, and pancreatic cancer. This breakthrough research offers new hope for cancer patients.
🌱 **The Miracle of IL-10** 🌱
The secretion of IL-10 promotes the proliferation and effector functions of CAR T cells, leading not only to the regression of solid tumors but also inducing stem cell-like memory responses in lymphoid organs, providing enduring protection against tumor re-attack. Specifically, IL-10 HER2 CAR-T cells achieved complete regression of MC38-HER2 tumors in mice, with a cure rate of 90%. In the case of melanoma, IL-10 TRP-1 CAR-T cells achieved a clearance rate of 60%, with significant success in treating the orthotopic B16F10 melanoma model.
🦠 **A Weapon against Relapse** 🦠
In addition to complete regression of solid tumors, IL-10 CAR-T cells demonstrated the ability to prevent relapse in immunodeficient mice. Mice treated with IL-10 CD19 hCAR-T cells for Raji or PANC1-CD19 tumors exhibited complete tumor regression without relapse, indicating stronger anti-tumor capabilities of IL-10 CAR-T cells in xenograft models. Particularly noteworthy is the effective elimination of pancreatic ductal adenocarcinoma (PDAC) tumors by IL-10 CD19 hCAR-T cells, resulting in complete response in all treated mice.
💊 **A Revolutionary Treatment Approach** 💊
These findings suggest that IL-10-expressing CAR-T cells are an effective immunotherapy against various solid tumors, capable of achieving complete regression in multiple synthetic and xenograft tumor models. What’s more exciting is that preliminary results indicate the metabolism-enhanced IL-10 CD19 CAR-T cell therapy developed by Leman Biotech requires extremely low treatment doses, consistently achieving complete remission in numerous relapsed/refractory lymphoma or leukemia patients, paving the way for a new era in cancer treatment.
✨ **A Beacon of Hope** ✨
This breakthrough study brings hope to cancer patients and demonstrates the immense potential of CAR-T therapy in cancer treatment. Looking ahead, further advancements in this technology promise to provide more opportunities for recovery and survival to patients worldwide. Let’s anticipate more breakthroughs together and strive towards conquering cancer
#all #CARTtherapy #CancerTreatment #RRMM #IL10 #Tumor #Nature #MedicalBreakthrough #CARTCELL #coloncancer #breastcancer #melanoma #pancreatic 🌟🔬💊
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

A significant breakthrough in Chinese solid tumor CAR-T therapy, resulting in complete remission for pancreatic cancer patients.

