Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

**Breakthrough Chinese Monoclonal ADC Targets Nectin-4: Achieving 91.9% Disease Control Rate Across Multiple Solid Tumors**
**Breakthrough Chinese Monoclonal ADC Targets Nectin-4: Achieving 91.9% Disease Control Rate Across Multiple Solid Tumors**

Monoclonal ADC
#MonoclonalADC #Nectin4 #Solidtumor #9MW2821 #cervicalcancer #urothelialcarcinoma #esophagealcancer #breastcancer
Meet 9MW2821, a groundbreaking monoclonal antibody-drug conjugate (ADC) developed in China that targets Nectin-4, an adhesion molecule highly expressed in several solid tumors, including cervical cancer (CC), urothelial carcinoma (UC), esophageal cancer (EC), and breast cancer. Recently, this innovative therapy was granted Breakthrough Therapy Designation by the China Center for Drug Evaluation (CDE) for treating locally advanced or metastatic urothelial carcinoma, specifically in patients who have previously failed platinum-based chemotherapy and PD-(L)1 inhibitors.
The latest clinical results were unveiled at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, showcasing the remarkable efficacy of 9MW2821 in a Phase 1/2 trial involving 260 patients with various advanced solid tumors. These included UC, triple-negative breast cancer (TNBC), EC, and CC. Among 37 evaluable UC patients, the disease control rate (DCR) soared to an impressive 91.9%, with an objective response rate (ORR) of 62.2%. Additionally, the median overall survival (mOS) reached 14.2 months, while the median progression-free survival (mPFS) was 8.8 months.
This promising therapy is poised to redefine treatment paradigms for patients with advanced solid tumors who have limited options, marking a significant milestone in the global oncology landscape.

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp:+8613717959070
Email: doctor.huang@globecancer.com
#OncologyBreakthrough #CancerTreatment #Nectin4 #ADC #ClinicalResearch #Immunotherapy #UC #TNBC #CancerAwareness #BiotechInnovation
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

AACR 2024/Breakthrough Chinese Research – Monotherapy for Late-stage #SolidTumors and #Lymphomas
 AACR 2024/Breakthrough Chinese Research – Monotherapy for Late-stage #SolidTumors and #Lymphomas
AACR 2024/Breakthrough Chinese Research – Monotherapy for Late-stage #SolidTumors and #Lymphomas

AACR2024
#AACR2024
#TQB2916
#CD40
#ECOG
#Realcase
#Lymphomas
#Conclusion
 TQB2916 achieves CD40 engagement and immune activation through cytokine modulation. With its promising safety and efficacy profile, 200mg has been identified as the preliminary expansion dose. Studies exploring TQB2916 in combination with immune checkpoint inhibitors and/or other anti-cancer therapies are currently underway.
TQB2916 achieves CD40 engagement and immune activation through cytokine modulation. With its promising safety and efficacy profile, 200mg has been identified as the preliminary expansion dose. Studies exploring TQB2916 in combination with immune checkpoint inhibitors and/or other anti-cancer therapies are currently underway.
 Stay tuned for more updates on this groundbreaking treatment!
Stay tuned for more updates on this groundbreaking treatment!
 doctor.huang@globecancer.com
doctor.huang@globecancer.com
 WhatsApp +86137 1795 9070
WhatsApp +86137 1795 9070
The Medical Department will contact you as soon as they receive the reports.
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
TIL Therapy: Revolutionizing Cancer Treatment Worldwide! China Accelerates into the Fast Lane!
🌟 **TIL Therapy: Revolutionizing Cancer Treatment Worldwide! 🌎** China Accelerates into the Fast Lane!
📈 The number of cell immunotherapy trials for solid tumor treatment has surged globally, with a staggering growth from 66 in 2020 to 140 in 2022, a remarkable 110% increase! Particularly, TIL therapy trials have seen a remarkable surge, now accounting for 17.1% of all solid tumor treatments, sparking a new trend in cancer therapy that’s capturing the attention of researchers and companies alike.
🎯 TIL therapy boasts natural advantages in solid tumor treatment, boasting rich tumor-specific target points, robust tumor infiltration capabilities, and minimal side effects.
🔬 On February 16, 2024, the U.S. FDA officially greenlights Iovance Biotherapeutics’ lifileucel therapy for unresectable or metastatic melanoma, a groundbreaking personalized T-cell therapy using TIL sourced from the patient’s body.
FDA
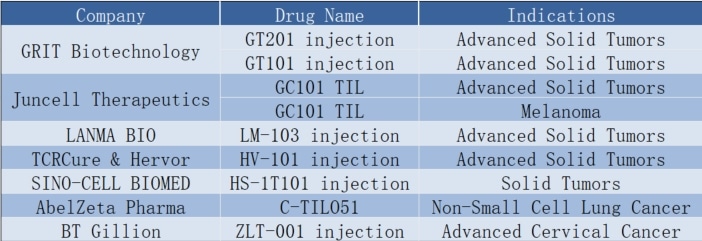
🚀 In early 2022, China’s Grit Biotechnology’s GT101 injection became the first TIL product to enter clinical trials, signaling China’s entry into the global TIL therapy scene. With players like Juncell Therapeutics, LANMA BIO, and BT Gillion joining the fray, Chinese TIL therapy is on the fast track to development, exploring various indications including melanoma, cervical cancer, lung cancer, and more!

TIL therapy
💰 According to Sullivan’s forecast, the global CGT market is set to skyrocket to $22 billion by 2025, with TIL therapy leading the charge.
💡 Chinese TIL therapy is paving its own path to commercialization and industrialization, offering cost-effective treatments compared to the U.S. Stay tuned for more innovations in cancer therapy from China! 🇨🇳
🌟We have access to medical resources from all cancer hospitals in China and can assist patients in receiving treatment from the best and top-tier oncologists in the CHINA.
doctor.huang@globecancer.com
WhatsApp+8613717959070
#TILTherapy #CancerTreatment #Innovation #MedicalBreakthroughs #ChinaHealthcare #GlobalHealthcare #CancerResearch 🚀🔬
