Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

The World’s First! China’s Non-Viral Site-Specific Integrated PD1-CAR-T Therapy Brings New Hope for Systemic Lupus Erythematosus(SLE) Patients
**The World’s First! China’s Non-Viral Site-Specific Integrated PD1-CAR-T Therapy Brings New Hope for Systemic Lupus Erythematosus(SLE) Patients**

SLE
#PD1 #CART #SLE #SystemicLupusErythematosus #Lupus #CARTTherapy #BRL203 #Nature
In November 2024, Chinese gene and cell therapy company BRL Medicine announced that its self-developed non-viral site-specific integrated PD1-CAR-T therapy, “BRL-203,” has received clinical trial approval from the National Medical Products Administration (NMPA) of China. This groundbreaking therapy aims to treat moderate to severe refractory systemic lupus erythematosus (SLE) and is the world’s first non-viral site-specific integrated CAR-T product approved for autoimmune disease treatment. This innovation brings new possibilities in lupus treatment and offers global patients a safer and more effective treatment option.
### Disruptive Treatment Brings Hope to Lupus Patients
Systemic lupus erythematosus (SLE) is a severe autoimmune disease that can lead to irreversible organ damage due to relapse and progression, severely affecting patients’ quality of life and even life expectancy. In recent years, SLE has garnered widespread attention and discussion, highlighting the urgent need for new therapies to alleviate patients’ suffering and extend their lives. While existing treatments can control the disease to some extent, they still rely on long-term immunosuppressive drugs, with suboptimal effectiveness and a high risk of relapse.
BRL Medicine’s non-viral site-specific integrated PD1-CAR-T therapy, “BRL-203,” offers a new treatment option for SLE patients. As a novel CAR-T cell therapy, it is expected to open a new era in autoimmune disease treatment, providing patients with sustainable disease control and reducing the risk of relapse.
### Chinese Non-Viral CAR-T: Overcoming Safety and Efficiency Challenges of Traditional Therapies
BRL Medicine’s “BRL-203” utilizes self-innovated non-viral site-specific integration technology, employing CRISPR/Cas9 gene-editing to precisely target the PD1 site and insert CAR molecules that target CD19. This non-viral method avoids the potential tumorigenic risks of traditional viral vector production, significantly enhancing the safety of the product. Moreover, the therapy preparation takes only 3 days, greatly reducing the patient’s waiting time and lowering production costs, making it more accessible and affordable for patients.
Compared to traditional CAR-T products, which face challenges such as long production cycles, high costs, and potential risks, BRL Medicine’s non-viral site-specific integrated PD1-CAR-T platform not only enhances safety but also significantly improves clinical applicability. Previously, BRL Medicine’s other non-viral PD1-CAR-T therapy demonstrated excellent safety and efficacy in treating relapsed/refractory B-cell non-Hodgkin lymphoma (r/r NHL), with research results published in *Nature* magazine, earning widespread recognition in the medical community.
### Precise Targeting and Safe, Controllable Innovative Therapy
Unlike traditional random integration methods, China’s non-viral site-specific integration technology ensures that each CAR sequence is precisely placed at a designated location in the genome, reducing the risk of tumor formation during treatment. This breakthrough technology ensures product uniformity and significantly improves clinical safety. In early clinical trials, BRL Medicine’s PD1-CAR-T therapy achieved a 100% objective response rate (ORR), an 85.7% complete response rate (CR), with almost no high-risk side effects.
### Future Outlook: Diversified Options for Autoimmune Disease Treatment
The approval of BRL Medicine’s non-viral site-specific integrated PD1-CAR-T product is not only a boon for lupus patients but also a significant breakthrough in the global autoimmune disease treatment field. The company will accelerate the subsequent clinical progress of BRL-203, aiming to develop it into an efficient, safe, and accessible innovative therapy, bringing new hope to more patients.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#BRLMedicine #CAR_T #LupusTreatment #AutoimmuneDisease #NonViralTherapy #GeneTherapy #InnovativeMedicine #SLE #ClinicalTrials #MedicalBreakthrough
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China’s CAR-T Technology Leads the World: A Miraculous Recovery Journey for Children with systemic lupus erythematosus (SLE)
**China’s CAR-T Technology Leads the World: A Miraculous Recovery Journey for Children with systemic lupus erythematosus (SLE)**

LUPUS
#SLE #lupus #systemiclupuserythematosus #CAR_Ttherapy #CancerSurvivor #ChineseHealthcare#PatientStory
In the field of medicine, the boundary between science and miracles is often blurred. This October, news from Zhejiang, China, shocked the world: 20 children suffering from refractory systemic lupus erythematosus (SLE) successfully discontinued the use of steroids and all immunosuppressants after receiving CAR-T cell therapy.
This global breakthrough not only brings hope to patients and their families but also demonstrates the immense potential of CAR-T therapy in the treatment of autoimmune diseases.
### Immune System Reset, New Life Begins
Systemic lupus erythematosus (SLE) is a complex autoimmune disease that many patients struggle to control despite long-term reliance on medications. This clinical trial, led by a team of renowned Chinese medical experts, marks the first time autologous CD19-targeted CAR-T cells have been applied to treat pediatric SLE patients. In just seven months, the symptoms of all 20 children improved significantly, and their quality of life greatly increased.
These children ranged in age from 6 to 19 years, with disease durations varying from four months to 11 years. Prior to receiving CAR-T treatment, they had been on an average of more than five different medications, with some taking as many as nine, yet the results were less than ideal. The introduction of CAR-T therapy became their last hope.
Although CAR-T therapy has already seen widespread application in the field of hematologic malignancies, this treatment for pediatric SLE represents a new frontier for CAR-T technology in autoimmune disease treatment, paving the way for innovative medical exploration.
### Clinical Data: Miracles in Reality
Among the 20 children, many showed remarkable recovery. For example, a 12-year-old girl from Shanghai, who had experienced recurrent lupus flare-ups, with a “butterfly rash” covering her face and massive proteinuria, saw a dramatic improvement after receiving CAR-T therapy. Within six months, she stopped all medications, her proteinuria decreased significantly, and her facial rash completely disappeared. By September, she had returned to school and was living almost normally.
Another 15-year-old girl from Shandong, despite having been diagnosed only two years prior, had progressed rapidly to kidney failure, requiring dialysis. After treatment, her blood creatinine level dropped from 700 μmol/L to 170 μmol/L, and her urine output increased from 50ml to 1000ml, allowing her to fully stop dialysis.
Moreover, all 20 children successfully discontinued steroids and immunosuppressants, and some patients’ disease activity scores dropped to zero. This means their immune systems have been reset, providing long-term control and, in some cases, a complete cure.
### Safety and Side Effects of CAR-T Therapy
Every revolutionary treatment carries risks, but the safety of China’s CAR-T therapy in this trial has been encouraging. CAR-T therapy works by genetically modifying T cells to recognize and attack specific target cells. Some patients experienced mild side effects, such as fever, nausea, and fatigue. Among the 20 children, only three exhibited mild neurological symptoms, which quickly improved with symptomatic treatment.
Research shows that CAR-T treatment in pediatric SLE patients comes with relatively mild side effects, and most patients did not experience any severe adverse reactions. This makes the future of CAR-T therapy in treating refractory lupus even more promising. This technology not only delivers significant therapeutic effects but also restores hope for a better future for these children.
### A Forward-Looking Treatment: From SLE to Other Diseases
Following the success of these 20 SLE cases, the research team plans to extend the application of China’s CAR-T technology to other autoimmune diseases, such as ANCA-associated vasculitis (AAV) and multidrug-resistant steroid-resistant nephrotic syndrome (MDR-SRNS). This means that in the future, more patients will have the opportunity to benefit from this revolutionary therapy.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CancerTreatment #Immunotherapy #MedicalBreakthrough #AdvancedMedicine #HealthRecovery
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

**Breakthrough in China’s CAR-T Therapy: 13-Year-Old Girl Overcomes Lupus, Opening New Hope for a Cure**
**Breakthrough in China’s CAR-T Therapy: 13-Year-Old Girl Overcomes Lupus, Opening New Hope for a Cure**

Lupus
#LupusTreatment #HopeForLupus #lupus #AutoimmuneDisease #ChinaHealthcare #SystemicLupusErythematosus #Erythematosus #patientstory #SLE
In June 2024, 13-year-old Qingqing experienced a major turning point in her life. A year after being diagnosed with systemic lupus erythematosus (SLE), she received innovative CAR-T therapy at Shanghai Children’s Medical Center and successfully achieved disease remission. SLE, often referred to as the “incurable cancer,” is an autoimmune disease that affects millions of people worldwide, severely damaging the brain, lungs, kidneys, and blood system. Even when not in an acute flare, patients often suffer from chronic symptoms such as fatigue, rashes, pain, and fever.
Qingqing’s treatment marks a significant breakthrough in the global fight against SLE. Previously, SLE patients could only rely on lifelong medications, such as steroids and immunosuppressants, to control the disease and prevent severe complications. However, these drugs often come with side effects and long-term organ damage. Qingqing’s case has offered the world new hope. A team led by Dr. Li Benshang, chief physician of the Hematology and Oncology Department, and Dr. Yin Lei, head of the Nephrology Department, pioneered the use of CAR-T therapy in SLE treatment and initiated a clinical study. After just two months of treatment, Qingqing’s condition has fully gone into remission, and all medications were discontinued.
CAR-T therapy was originally used in cancer treatment by extracting a patient’s T cells and genetically modifying them to recognize and kill abnormal cells in the body. In the treatment of SLE, CAR-T therapy eliminates the abnormal plasma cells producing autoantibodies, effectively “resetting” the patient’s immune system and addressing the root cause of the disease.
Qingqing’s success story is not only a milestone in China’s CAR-T technology but also a major leap forward in the global treatment of autoimmune diseases like SLE. Since the first CAR-T treatment cured an SLE patient in 2021, numerous clinical trials have been conducted worldwide, and several Chinese hospitals have also achieved success. However, while early results are encouraging, further research and validation are needed to confirm the long-term safety and efficacy of the treatment.
With 20% of the global population affected by various types of autoimmune diseases, CAR-T therapy holds the potential to bring new life to millions of patients. China’s ongoing research and international collaboration in this field offer unprecedented hope for overcoming persistent diseases like SLE.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
(Https://wa.me/+8613717959070)
Email: doctor.huang@globecancer.com
#CAR-Ttherapy #LupusTreatment #AutoimmuneDisease #MedicalInnovation #ChinaHealthcare #Immunotherapy #CancerTreatment #HealthBreakthrough #HopeForLupus #FutureOfMedicine
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

“Curing” the Untouchable Cancer – Systemic Lupus Erythematosus: Chinese Experts Take a Major Leap Forward
**”Curing” the Untouchable Cancer – Systemic Lupus Erythematosus: Chinese Experts Take a Major Leap Forward**

Lupus
#LupusTreatment #HopeForLupus #lupus #AutoimmuneDisease #ChinaHealthcare #SystemicLupusErythematosus #Erythematosus
Once hailed as a revolutionary therapy in the field of oncology, Chimeric Antigen Receptor T-cell (CAR-T) therapy is now demonstrating extraordinary potential in the treatment of Systemic Lupus Erythematosus (SLE). For this troublesome autoimmune disease that has long plagued patients and doctors alike, CAR-T therapy may be the dawn of ending this struggle.
In 2021, researchers in Germany successfully treated a 20-year-old woman with severe SLE using CAR-T therapy for the first time. While CAR-T therapy had already achieved significant success in treating hematologic malignancies, it is now opening new doors for autoimmune disease treatments. Since then, over a dozen global clinical trials have been initiated to explore CAR-T’s potential for SLE, and initial results are promising.
**How Does CAR-T Treat Lupus?**
CAR-T therapy involves extracting the patient’s T-cells, genetically modifying them to recognize and destroy harmful cells, and then reinfusing them into the body. For cancer patients, this means eliminating tumor cells; for SLE patients, it means clearing the abnormal plasma cells that produce self-antibodies, thus fundamentally curing the disease.
In China, researchers are also accelerating the application of CAR-T therapy. In June and July of 2024, two patients in Shanghai received CAR-T treatment and achieved significant results. A young girl named Qingqing and an adult woman, Ms. S, saw dramatic improvements in their symptoms and discontinued all medications related to their primary illness. Laboratory test results also showed marked improvement.
“Two months after treatment, the patients’ proteinuria disappeared, and the SLE Disease Activity Index score was 0, indicating complete remission,” said nephrologist Dr. Yin Lei. “They have returned to their normal lives.”
**Breakthroughs in Autoimmune Disease Treatment**
The success of CAR-T therapy in treating SLE is not only a boon for lupus patients but also brings new hope to millions of other autoimmune disease sufferers worldwide. Currently, about 20% of the global population is affected to varying degrees by over 100 different autoimmune diseases. The advent of CAR-T therapy could revolutionize the treatment landscape for these conditions.
Despite the promising outlook, experts remain cautious. Long-term safety and efficacy still require more research to confirm. Additionally, technical challenges such as improving the quality of T-cells after immunosuppressive therapy need to be addressed.
Nevertheless, the future looks very promising. CAR-T therapy may represent the best opportunity to overcome autoimmune diseases, including lupus.
**A Brighter Future for Lupus Patients**
Although the application of CAR-T therapy in SLE is still in its early stages, preliminary results have already brought significant hope. Future research and clinical trials will help us better understand its long-term impact and further refine the technology. For those who once faced incurable diseases, the future may no longer be so bleak.
Global pharmaceutical companies are also shifting their priorities from cancer treatment to research on autoimmune diseases. As CAR-T therapy continues to advance, more patients will benefit, bringing new hope for many.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
(Https://wa.me/+8613717959070)
Email: doctor.huang@globecancer.com
#CAR-Ttherapy #LupusTreatment #AutoimmuneDisease #MedicalInnovation #ChinaHealthcare #Immunotherapy #CancerTreatment #HealthBreakthrough #HopeForLupus #FutureOfMedicine
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese CAR-T Therapy: A New Frontier in Treating Systemic Lupus Erythematosus
Chinese CAR-T Therapy: A New Frontier in Treating Systemic Lupus Erythematosus

Systemic Lupus Erythematosus
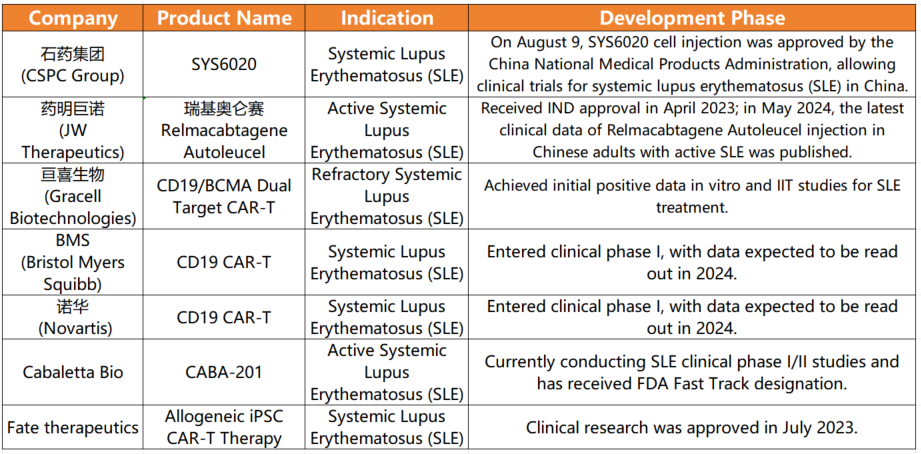
Recently, CSPC PHARMA’s CAR-T product, SYS6020, received approval for clinical trials from China’s National Medical Products Administration (NMPA). This groundbreaking development represents a significant milestone in cell therapy, especially for treating refractory active systemic lupus erythematosus (SLE). Notably, SYS6020 targets the BCMA antigen, potentially providing a novel and cost-effective treatment option for SLE patients worldwide.
**Innovative Approach and Promising Safety Profile**
SYS6020 is a pioneering mRNA-LNP-based CAR-T cell injection, marking it as the first of its kind to enter clinical trials. This innovative therapy specifically recognizes the BCMA antigen, attacking and eliminating immune cells responsible for elevated autoantibodies. This approach offers a unique, safe, and effective treatment option for SLE, distinct from traditional CAR-T therapies. Notably, SYS6020 exhibits high cell viability, a high CAR-positive rate, and reduced risks of genomic integration and cytokine release syndrome (CRS), common issues with conventional CAR-T treatments.
Preclinical studies have shown that SYS6020 effectively kills BCMA antigen-positive myeloma cells while maintaining a favorable safety profile. By employing LNP for T cell transfection, the therapy significantly reduces costs compared to using lentiviral vectors, lessening the financial burden on patients.
Lupus
