Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China`s Breakthrough TIL Therapy GT201 Shows Remarkable Results in Advanced Solid Tumor Treatment: 100% Disease Control Rate, 69% Tumor Reduction
**China`s Breakthrough TIL Therapy GT201 Shows Remarkable Results in Advanced Solid Tumor Treatment: 100% Disease Control Rate, 69% Tumor Reduction**

TIL Therapy
#TILTherapy #GT201 #SolidTumor #TIL #ASCO2024
Recent research results indicate that the next-generation gene-edited TIL (Tumor-Infiltrating Lymphocyte) therapy, GT201, independently developed by Chinese biotech company GRIT Biotechnology, has shown significant potential in combating various advanced solid tumors. Through a unique membrane-bound cytokine design and the advanced retroviral system StaViral, GT201 has successfully overcome the traditional TIL therapy`s excessive reliance on IL-2, significantly improving its efficacy and durability in cancer treatment.
At the 2024 ASCO conference, the research team presented the results of the Phase I clinical trial for GT201. A total of 7 patients with advanced solid tumors, including non-small cell lung cancer, malignant melanoma, cervical cancer, and ovarian cancer, were enrolled in the trial. These patients had previously undergone at least three different treatments and showed notable efficacy after receiving GT201.
The data revealed that, among the 7 evaluable patients, 3 (42.9%) achieved confirmed partial responses (PR), with one patient’s tumor shrinking by an impressive 69%. Additionally, 2 patients (28.6%) achieved stable disease (SD). Notably, all 3 patients in the non-small cell lung cancer subgroup achieved disease control (SD ≥ 24 weeks or PR), resulting in a 100% disease control rate. These findings not only highlight the efficacy of GT201 but also bring new hope to patients with advanced non-small cell lung cancer.
Another significant advantage of GT201 is its safety profile. Throughout the trial, the therapy demonstrated good tolerability with manageable side effects. This result suggests that GT201 has the potential to become a powerful and effective treatment option for patients with advanced solid tumors, especially in the field of non-small cell lung cancer, where its objective response rate and disease control durability are highly encouraging.
As the clinical trials of GT201 continue to progress, it is poised to lead the future of solid tumor treatment and further advance TIL therapies. We look forward to more patients benefiting from this groundbreaking treatment.
To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070(Https://wa.me/+8613717959070)
Email: doctor.huang@globecancer.com
#SolidTumorTreatment #NonSmallCellLungCancer #CancerImmunotherapy #GRITBiotechnology #CancerResearch #GeneEditing #CancerTreatmentInnovation
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
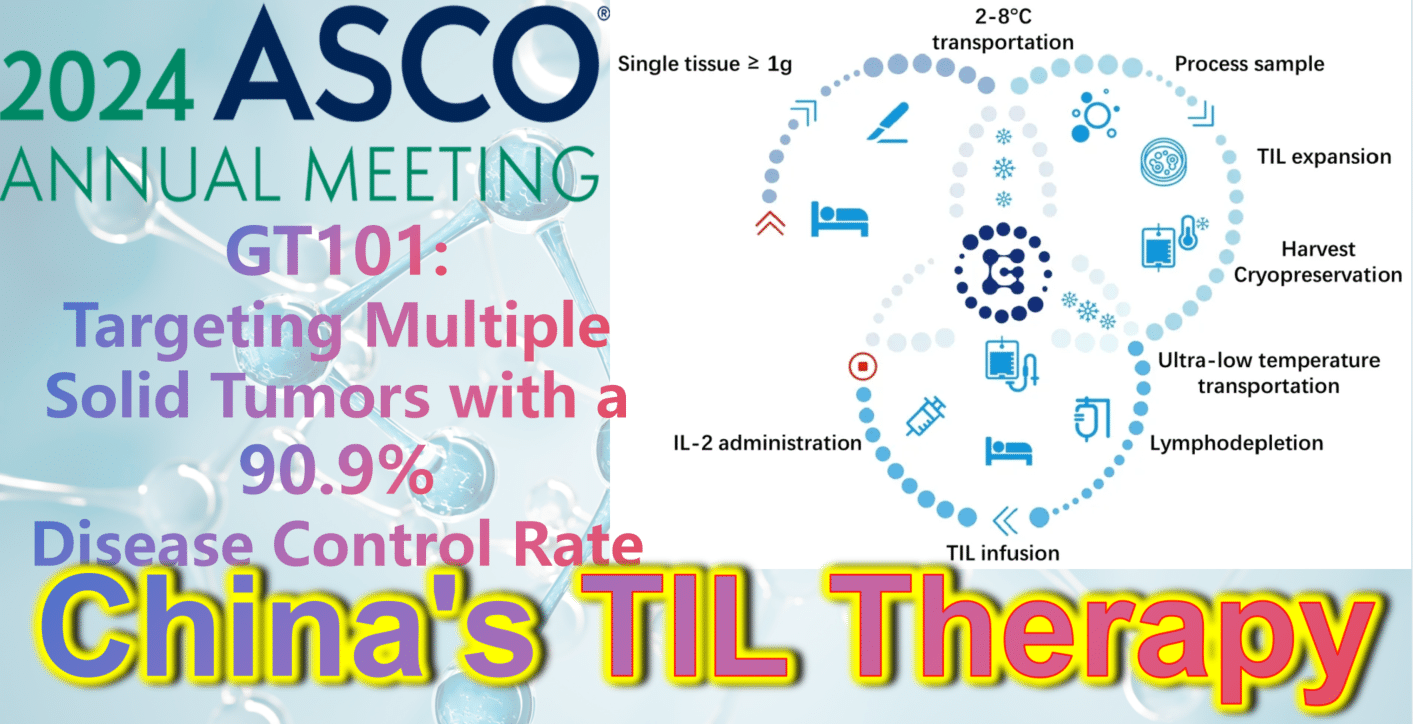
2024 ASCO China Highlights: China’s Indigenous TIL Therapy – GT101: Targeting Multiple Solid Tumors with a 90.9% Disease Control Rate
**2024 ASCO China Highlights: China’s Indigenous TIL Therapy Makes a Strong Debut, Targeting Small Cell Lung Cancer, Melanoma, Cervical Cancer, with a DCR Over 90%**
**GT101: Targeting Multiple Solid Tumors with a 90.9% Disease Control Rate**

TIL Therapy
GT101, independently developed by Gravel Biotech, is an autologous tumor-infiltrating lymphocyte (TIL) therapy. On April 22, 2022, its clinical trial implied consent was approved by the National Medical Products Administration (NMPA) (Acceptance No.: CXSL2200061). It is indicated for treating various solid tumors including non-small cell lung cancer, melanoma, and cervical cancer. Notably, GT101 is China’s first approved clinical TIL cell therapy and holds promise as the first cell therapy to conquer solid tumors!
Recently, at the 2024 ASCO Annual Meeting, results from the Phase 1 clinical trial of GT101 TIL therapy (NCT05430373) were announced. By November 10, 2023, a total of 14 patients with recurrent or metastatic solid tumors (including small cell lung cancer, melanoma, cervical cancer) had been enrolled, all with an ECOG performance status of 0 or 1 and having received a median of 2.6 prior lines of therapy. After enrollment, tumor tissue was obtained via appropriate surgical procedures for GT101 preparation, followed by approximately 30 days of TIL cell culture. Patients then underwent non-myeloablative lymphocyte depletion (cyclophosphamide + fludarabine), GT101 reinfusion therapy, and IL-2 (Interleukin-2) treatment.
1. **All enrolled patients (n=14):** The Objective Response Rate (ORR) was 35.7%. Among them, 28.6% (4 patients) achieved partial response (PR), 57.1% (8 patients) achieved stable disease (SD), and 7.1% (1 patient) achieved complete response (CR).
2. **In cervical cancer patients (n=11):** The Objective Response Rate (ORR) reached 45.5% (5/11). The Disease Control Rate (DCR) was as high as 90.9% (10/11), with 36.4% (4 patients) achieving partial response (PR) and 9.1% (1 patient) achieving complete response (CR). The median Progression-Free Survival (PFS) was 4.2 months. According to Kaplan-Meier statistics, the durations of complete response (CR) and progression-free survival (PFS) were 24 weeks and 36 weeks, respectively.
In conclusion, GT101 demonstrated promising clinical efficacy and manageable safety in combination with lymphocyte depletion and high-dose IL-2 treatment. Particularly in the treatment of cervical cancer, its objective response rate and duration of response are remarkable!
**How to Seek Help from TIL Therapy?**
The good news is that several TIL therapy clinical trials are currently recruiting in China, primarily targeting various solid tumors such as non-small cell lung cancer, melanoma, cholangiocarcinoma, esophageal squamous cell carcinoma, head and neck squamous cell carcinoma, breast cancer, ovarian cancer, cervical cancer, endometrial cancer, fallopian tube cancer, urothelial cancer, and renal cancer.
Patients seeking help from TIL therapy can submit their complete treatment history, recent pathology reports, imaging examination reports, and discharge summaries to Advanced Medicine in China
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#ASCO2024 #TILTherapy #GT101 #CancerResearch #SolidTumors #ClinicalTrials #LungCancer #Melanoma #CervicalCancer #Immunotherapy #ChinaBiotech #CancerTreatment
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
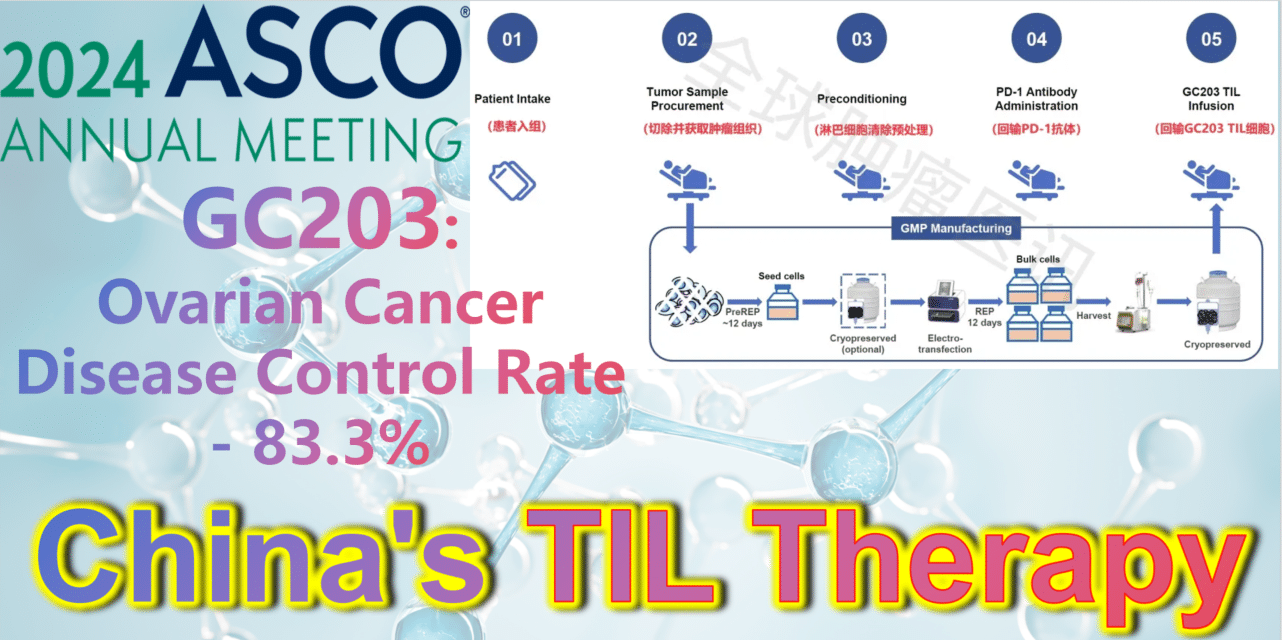
2024 ASCO China Voice: China’s TIL therapy – GC203: A Powerful Strike Against Ovarian Cancer with an 83.3% Disease Control Rate
2024 ASCO China Voice: China’s TIL therapy makes a grand debut, targeting ovarian cancer.
**GC203: A Powerful Strike Against Ovarian Cancer with an 83.3% Disease Control Rate**

TIL therapy
Ovarian cancer is a type of gynecologic tumor with a poor prognosis, with 70% of patients being diagnosed at a late stage. Unfortunately, effective treatment options for advanced ovarian cancer are quite limited, primarily relying on platinum-based chemotherapy. However, many ovarian cancer patients are not responsive to chemotherapy. Thus, there is an urgent need for new treatment options.
GC203 (mbIL-7-TIL) is a novel non-viral vector gene-modified TIL therapy utilizing membrane-bound IL-7. Developed by JunSai Biotech using the DeepTIL® cell expansion platform and NovaGMP® gene modification platform, it efficiently modifies T cells in a more economical way, enhancing the antitumor activity of TIL cells, activating internal immune cells, and avoiding systemic toxicity. It does not require lymphodepletion or combined IL-2 therapy post-infusion. A single patient is expected to save approximately 150,000 RMB in associated clinical costs, significantly improving the accessibility of TIL therapy and benefiting more cancer patients.
At the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, the latest clinical study results of GC203’s Phase 1 trial (NCT05468307) were announced. Between September 2021 and January 2024, 20 patients with recurrent ovarian cancer were enrolled, having undergone a median of 2.5 lines (range 1-9) of chemotherapy regimens (including PARP inhibitors, immune checkpoint inhibitors, etc.). After enrollment, patients first underwent tumor tissue resection, which was transported to GMP for a 22-26 day preparation period. The cryopreserved infusion products were then returned to the clinical center. Finally, patients received lymphocyte depletion pretreatment (including cyclophosphamide and hydroxychloroquine), a one-time PD-1 antibody infusion, and GC203 TIL cell reinfusion therapy.
After a median follow-up of 8.7 months (range, 2.9-18.8 months), results from 18 evaluable patients showed the following:
-
**Objective Response Rate (ORR):** The ORR in evaluable patients (n=18) was 33.3% (95% CI: 16.3%-56.3%). Among them, 22.2% (4 patients) achieved partial response (PR), and 11.1% (2 patients) achieved complete response (CR).
-
**Disease Control Rate (DCR):** The DCR in evaluable patients (n=18) was 83.3% (95% CI: 60.8%-94.2%).
-
**Median Progression-Free Survival (PFS):** The median PFS was 5.5 months (range, 1.0-14.1 months).
-
**Overall Survival (OS) Rate:** The 6-month OS rate was 75.6% (95% CI: 57.4%-99.6%); the 12-month OS rate was 68.8% (95% CI: 49.3%-95.9%).
-
**Adverse Reactions:** Most treatment-emergent adverse events (TEAEs) were grade 1 or 2, with common adverse reactions including elevated C-reactive protein levels (33%), fever (33%), and fatigue (11%), which could be alleviated or cured with symptomatic treatment. No other serious adverse reactions were observed.
In summary, for patients with recurrent or metastatic ovarian cancer with limited treatment options, GC203 TIL cell reinfusion therapy has shown good efficacy. Due to low-intensity pretreatment and no need for combined IL-2 therapy, its safety is significantly improved compared to traditional TIL therapy.
**How to Seek Help from TIL Therapy?**
The good news is that several TIL therapy clinical trials are currently recruiting in China, primarily targeting various solid tumors such as non-small cell lung cancer, melanoma, cholangiocarcinoma, esophageal squamous cell carcinoma, head and neck squamous cell carcinoma, breast cancer, ovarian cancer, cervical cancer, endometrial cancer, fallopian tube cancer, urothelial cancer, and renal cancer.
Patients seeking help from TIL therapy can submit their complete treatment history, recent pathology reports, imaging examination reports, and discharge summaries to Advanced Medicine in China.
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#OvarianCancer #TILTherapy #CancerTreatment #ASCO2024 #GC203 #Immunotherapy #MedicalResearch #Biotech #Oncology #ClinicalTrials #CancerInnovation #JunSaiBiotech #TIL #CancerBreakthrough #PatientCare #MedicalAdvancements
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

No Tumor Recurrence for Nearly 3 Years, China’s National Research #TIL Therapy #GC101 Targets Various Solid Tumors!
 No Tumor Recurrence for Nearly 3 Years, China’s National Research #TIL Therapy #GC101 Targets Various Solid Tumors!
No Tumor Recurrence for Nearly 3 Years, China’s National Research #TIL Therapy #GC101 Targets Various Solid Tumors!

TIL Therapy
 In recent years, immunotherapy has emerged as a pivotal direction in the future of cancer treatment. Tumor-infiltrating lymphocytes (#TILs) are hailed as the “natural enemies” of cancer cells. TIL therapy developed based on patients’ own tumor-infiltrating lymphocytes boasts advantages such as rich tumor-specific targets, excellent tumor homing capabilities, strong infiltration abilities, and a high safety profile.
In recent years, immunotherapy has emerged as a pivotal direction in the future of cancer treatment. Tumor-infiltrating lymphocytes (#TILs) are hailed as the “natural enemies” of cancer cells. TIL therapy developed based on patients’ own tumor-infiltrating lymphocytes boasts advantages such as rich tumor-specific targets, excellent tumor homing capabilities, strong infiltration abilities, and a high safety profile.
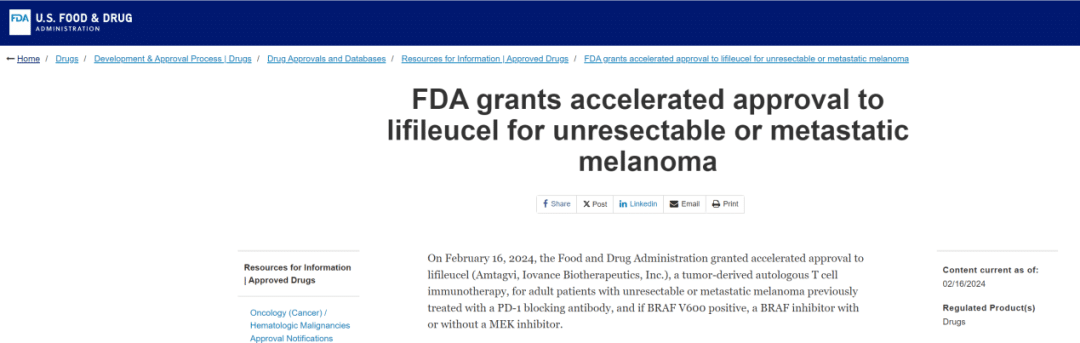
 On February 16, 2024, #Iovance Biotherapeutics announced the #FDA approval of Lifileucel (LN-144) for the treatment of advanced melanoma progressing after PD-1 antibody therapy, marketed as #AMTAGVI™. This marks the global first approval of a TIL therapy and the first approval worldwide for T-cell therapy for solid tumors, marking a significant milestone! #LN144
On February 16, 2024, #Iovance Biotherapeutics announced the #FDA approval of Lifileucel (LN-144) for the treatment of advanced melanoma progressing after PD-1 antibody therapy, marketed as #AMTAGVI™. This marks the global first approval of a TIL therapy and the first approval worldwide for T-cell therapy for solid tumors, marking a significant milestone! #LN144
 On the domestic front in China, several companies have announced good news regarding the clinical approval of TIL pipelines. Overall, the enthusiasm for TIL therapies is on the rise and increasingly intense. Today, let me introduce to you the first independently developed TIL cell therapy #GC101 from Juncell Therapeutics.
On the domestic front in China, several companies have announced good news regarding the clinical approval of TIL pipelines. Overall, the enthusiasm for TIL therapies is on the rise and increasingly intense. Today, let me introduce to you the first independently developed TIL cell therapy #GC101 from Juncell Therapeutics.
 With no tumor recurrence for nearly 3 years, GC01TIL is the first autologous natural tumor-infiltrating lymphocyte injection developed by Juncell Therapeutics, which officially obtained clinical trial implicit approval from the National Medical Products Administration (#NMPA) on April 24, 2022.
With no tumor recurrence for nearly 3 years, GC01TIL is the first autologous natural tumor-infiltrating lymphocyte injection developed by Juncell Therapeutics, which officially obtained clinical trial implicit approval from the National Medical Products Administration (#NMPA) on April 24, 2022.
 Distinguished from Iovance’s approved AMTAGVI™ (#Lifileucel), innovative GC101TIL therapy requires patients to be treated in ordinary wards without the need for high-intensity pre-conditioning before TIL cell infusion or any IL-2 dosage post-infusion. This simplified clinical protocol ensures effective TIL cell proliferation within patients, greatly avoiding the risks associated with AMTAGVI™’s black box warnings (such as treatment-related deaths, persistent severe cytopenia, severe infections, cardiopulmonary and renal function damage), substantially improving the safety, convenience, and accessibility of TIL therapy.
Distinguished from Iovance’s approved AMTAGVI™ (#Lifileucel), innovative GC101TIL therapy requires patients to be treated in ordinary wards without the need for high-intensity pre-conditioning before TIL cell infusion or any IL-2 dosage post-infusion. This simplified clinical protocol ensures effective TIL cell proliferation within patients, greatly avoiding the risks associated with AMTAGVI™’s black box warnings (such as treatment-related deaths, persistent severe cytopenia, severe infections, cardiopulmonary and renal function damage), substantially improving the safety, convenience, and accessibility of TIL therapy.
 Currently, according to the latest revelations from Juncell Therapeutics, the Phase I clinical trial of Juncell Therapeutics’ autologous natural TIL cell injection GC101 is underway in eight authoritative tertiary hospitals in China, achieving significant efficacy.
Currently, according to the latest revelations from Juncell Therapeutics, the Phase I clinical trial of Juncell Therapeutics’ autologous natural TIL cell injection GC101 is underway in eight authoritative tertiary hospitals in China, achieving significant efficacy.
 GC101, targeting various types of advanced solid tumors including malignant melanoma, cervical cancer, and lung cancer, has demonstrated an objective response rate of over 35%. Four patients have achieved complete tumor remission (#CR), with the longest no-recurrence survival period approaching 3 years, without experiencing any severe adverse reactions related to treatment, significantly improving the safety, applicability, and accessibility of #TILtherapy. Let us look forward to more surprises that GC101 will bring in the future!
GC101, targeting various types of advanced solid tumors including malignant melanoma, cervical cancer, and lung cancer, has demonstrated an objective response rate of over 35%. Four patients have achieved complete tumor remission (#CR), with the longest no-recurrence survival period approaching 3 years, without experiencing any severe adverse reactions related to treatment, significantly improving the safety, applicability, and accessibility of #TILtherapy. Let us look forward to more surprises that GC101 will bring in the future!
 Currently, #Juncell Therapeutics’ GC101 clinical trial for the treatment of advanced solid tumors is actively recruiting :
Currently, #Juncell Therapeutics’ GC101 clinical trial for the treatment of advanced solid tumors is actively recruiting :
FOR FREE
 Key Inclusion Criteria:
Key Inclusion Criteria:
1. Aged 18-75 years, regardless of gender;
2. Definitive diagnosis of malignant solid tumors (including but not limited to #melanoma, #lungcancer, #cervicalcancer, #esophagealsquamouscellcarcinoma, #headandnecksquamouscellcarcinoma, #endometrialcancer);
3. Standard treatment failure or lack of effective treatment options;
4. At least 2 lesions, with the physical condition supporting minimally invasive surgical sampling.
 This clinical trial is free of charge for participants. If you wish to participate in this clinical trial, you need to submit your treatment history, recent imaging and blood test reports, infectious disease reports, and discharge summaries to <@Advanced Medicine in China for preliminary assessment.
This clinical trial is free of charge for participants. If you wish to participate in this clinical trial, you need to submit your treatment history, recent imaging and blood test reports, infectious disease reports, and discharge summaries to <@Advanced Medicine in China for preliminary assessment.
 Email: doctor.huang@globecancer.com,
Email: doctor.huang@globecancer.com,
 WhatsApp: +8613717959070
WhatsApp: +8613717959070
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
TIL Therapy: Revolutionizing Cancer Treatment Worldwide! China Accelerates into the Fast Lane!
🌟 **TIL Therapy: Revolutionizing Cancer Treatment Worldwide! 🌎** China Accelerates into the Fast Lane!
FDA

TIL therapy
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
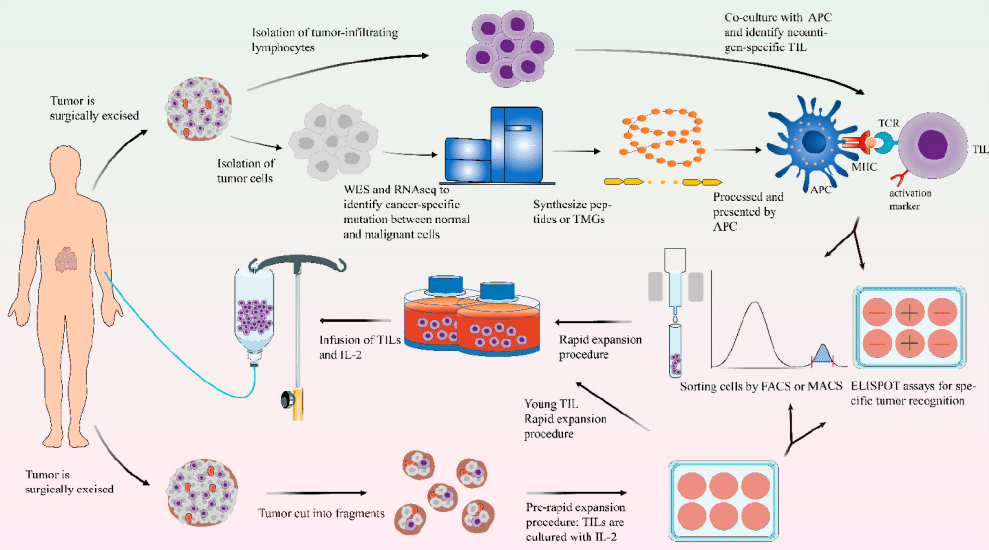
Unlocking the Potential: Understanding TIL Therapy for Solid Tumors
🔍 Unlocking the Potential: Understanding TIL Therapy for Solid Tumors🧬
💡What is TIL therapy?🧬
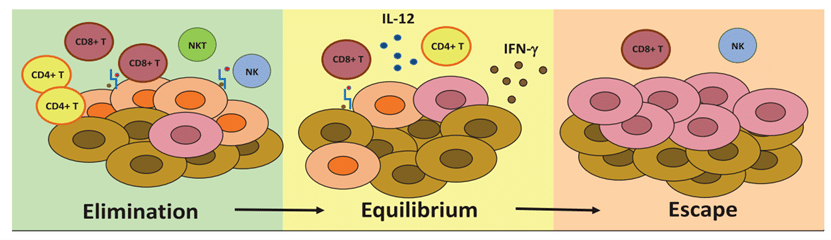
Cancer Development
Understanding Cancer Development: Immune Surveillance, Immune Equilibrium, and Immune Escape ���

TIL Therapy
