Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

No Tumor Recurrence for Nearly 3 Years, China’s National Research #TIL Therapy #GC101 Targets Various Solid Tumors!
 No Tumor Recurrence for Nearly 3 Years, China’s National Research #TIL Therapy #GC101 Targets Various Solid Tumors!
No Tumor Recurrence for Nearly 3 Years, China’s National Research #TIL Therapy #GC101 Targets Various Solid Tumors!

TIL Therapy
 In recent years, immunotherapy has emerged as a pivotal direction in the future of cancer treatment. Tumor-infiltrating lymphocytes (#TILs) are hailed as the “natural enemies” of cancer cells. TIL therapy developed based on patients’ own tumor-infiltrating lymphocytes boasts advantages such as rich tumor-specific targets, excellent tumor homing capabilities, strong infiltration abilities, and a high safety profile.
In recent years, immunotherapy has emerged as a pivotal direction in the future of cancer treatment. Tumor-infiltrating lymphocytes (#TILs) are hailed as the “natural enemies” of cancer cells. TIL therapy developed based on patients’ own tumor-infiltrating lymphocytes boasts advantages such as rich tumor-specific targets, excellent tumor homing capabilities, strong infiltration abilities, and a high safety profile.
 On February 16, 2024, #Iovance Biotherapeutics announced the #FDA approval of Lifileucel (LN-144) for the treatment of advanced melanoma progressing after PD-1 antibody therapy, marketed as #AMTAGVI™. This marks the global first approval of a TIL therapy and the first approval worldwide for T-cell therapy for solid tumors, marking a significant milestone! #LN144
On February 16, 2024, #Iovance Biotherapeutics announced the #FDA approval of Lifileucel (LN-144) for the treatment of advanced melanoma progressing after PD-1 antibody therapy, marketed as #AMTAGVI™. This marks the global first approval of a TIL therapy and the first approval worldwide for T-cell therapy for solid tumors, marking a significant milestone! #LN144
 On the domestic front in China, several companies have announced good news regarding the clinical approval of TIL pipelines. Overall, the enthusiasm for TIL therapies is on the rise and increasingly intense. Today, let me introduce to you the first independently developed TIL cell therapy #GC101 from Juncell Therapeutics.
On the domestic front in China, several companies have announced good news regarding the clinical approval of TIL pipelines. Overall, the enthusiasm for TIL therapies is on the rise and increasingly intense. Today, let me introduce to you the first independently developed TIL cell therapy #GC101 from Juncell Therapeutics.
 With no tumor recurrence for nearly 3 years, GC01TIL is the first autologous natural tumor-infiltrating lymphocyte injection developed by Juncell Therapeutics, which officially obtained clinical trial implicit approval from the National Medical Products Administration (#NMPA) on April 24, 2022.
With no tumor recurrence for nearly 3 years, GC01TIL is the first autologous natural tumor-infiltrating lymphocyte injection developed by Juncell Therapeutics, which officially obtained clinical trial implicit approval from the National Medical Products Administration (#NMPA) on April 24, 2022.
 Distinguished from Iovance’s approved AMTAGVI™ (#Lifileucel), innovative GC101TIL therapy requires patients to be treated in ordinary wards without the need for high-intensity pre-conditioning before TIL cell infusion or any IL-2 dosage post-infusion. This simplified clinical protocol ensures effective TIL cell proliferation within patients, greatly avoiding the risks associated with AMTAGVI™’s black box warnings (such as treatment-related deaths, persistent severe cytopenia, severe infections, cardiopulmonary and renal function damage), substantially improving the safety, convenience, and accessibility of TIL therapy.
Distinguished from Iovance’s approved AMTAGVI™ (#Lifileucel), innovative GC101TIL therapy requires patients to be treated in ordinary wards without the need for high-intensity pre-conditioning before TIL cell infusion or any IL-2 dosage post-infusion. This simplified clinical protocol ensures effective TIL cell proliferation within patients, greatly avoiding the risks associated with AMTAGVI™’s black box warnings (such as treatment-related deaths, persistent severe cytopenia, severe infections, cardiopulmonary and renal function damage), substantially improving the safety, convenience, and accessibility of TIL therapy.
 Currently, according to the latest revelations from Juncell Therapeutics, the Phase I clinical trial of Juncell Therapeutics’ autologous natural TIL cell injection GC101 is underway in eight authoritative tertiary hospitals in China, achieving significant efficacy.
Currently, according to the latest revelations from Juncell Therapeutics, the Phase I clinical trial of Juncell Therapeutics’ autologous natural TIL cell injection GC101 is underway in eight authoritative tertiary hospitals in China, achieving significant efficacy.
 GC101, targeting various types of advanced solid tumors including malignant melanoma, cervical cancer, and lung cancer, has demonstrated an objective response rate of over 35%. Four patients have achieved complete tumor remission (#CR), with the longest no-recurrence survival period approaching 3 years, without experiencing any severe adverse reactions related to treatment, significantly improving the safety, applicability, and accessibility of #TILtherapy. Let us look forward to more surprises that GC101 will bring in the future!
GC101, targeting various types of advanced solid tumors including malignant melanoma, cervical cancer, and lung cancer, has demonstrated an objective response rate of over 35%. Four patients have achieved complete tumor remission (#CR), with the longest no-recurrence survival period approaching 3 years, without experiencing any severe adverse reactions related to treatment, significantly improving the safety, applicability, and accessibility of #TILtherapy. Let us look forward to more surprises that GC101 will bring in the future!
 Currently, #Juncell Therapeutics’ GC101 clinical trial for the treatment of advanced solid tumors is actively recruiting :
Currently, #Juncell Therapeutics’ GC101 clinical trial for the treatment of advanced solid tumors is actively recruiting :
FOR FREE
 Key Inclusion Criteria:
Key Inclusion Criteria:
1. Aged 18-75 years, regardless of gender;
2. Definitive diagnosis of malignant solid tumors (including but not limited to #melanoma, #lungcancer, #cervicalcancer, #esophagealsquamouscellcarcinoma, #headandnecksquamouscellcarcinoma, #endometrialcancer);
3. Standard treatment failure or lack of effective treatment options;
4. At least 2 lesions, with the physical condition supporting minimally invasive surgical sampling.
 This clinical trial is free of charge for participants. If you wish to participate in this clinical trial, you need to submit your treatment history, recent imaging and blood test reports, infectious disease reports, and discharge summaries to <@Advanced Medicine in China for preliminary assessment.
This clinical trial is free of charge for participants. If you wish to participate in this clinical trial, you need to submit your treatment history, recent imaging and blood test reports, infectious disease reports, and discharge summaries to <@Advanced Medicine in China for preliminary assessment.
 Email: doctor.huang@globecancer.com,
Email: doctor.huang@globecancer.com,
 WhatsApp: +8613717959070
WhatsApp: +8613717959070
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
Unlocking the Potential: Understanding TIL Therapy for Solid Tumors
🔍 Unlocking the Potential: Understanding TIL Therapy for Solid Tumors🧬
💡What is TIL therapy?🧬
According to the immune editing theory, during the process of tumor development, the body’s immune cells always play a role in killing tumor cells. Different immune cells (mainly lymphocytes) are transported to the tumor site, and these lymphocytes that have infiltrated into the tumor tissue and exerted cytotoxic effects are called tumor-infiltrating lymphocytes (TILs), including T cells, B cells, NK cells, macrophages, and various mononuclear and multinuclear immune cells, which can recognize, resist, attack, and kill tumors.
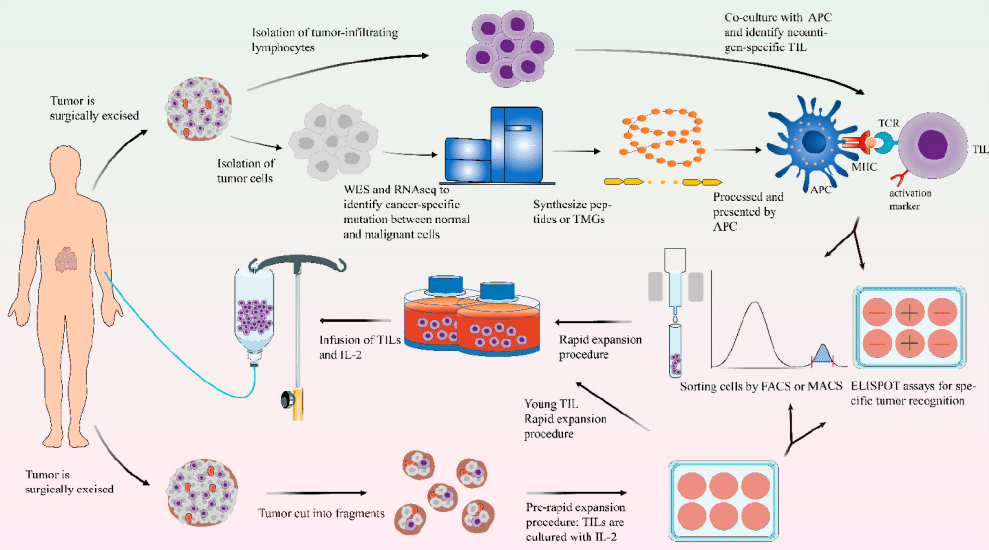
TIL therapy belongs to Cellular Immunotherapy, which is a method of treating using immunocytes that are activated, proliferated, or genetically engineered. The main steps include isolating specific immune-active cells (including T cells, NK cells, DC cells, and macrophages) from tumor patients, genetic modification or amplification in vitro, functional identification, and finally, returning for treatment.
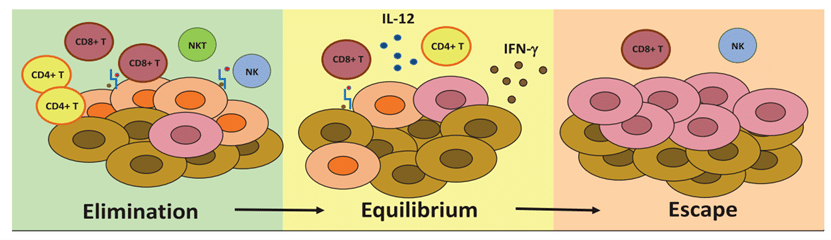
Cancer Development
Understanding Cancer Development: Immune Surveillance, Immune Equilibrium, and Immune Escape ���
TIL therapy refers to isolating tumor-infiltrating lymphocytes from tumor tissue, culturing them in vitro, expanding them massively, and then reinfusing them into the body for treatment.

TIL Therapy
🔬 The main routine steps of TIL therapy include:
1. Obtaining patient tumor tissue: mainly obtained through surgery or biopsy to include TIL cells.
2. Grinding tumor tissue: This step makes it easier for T lymphocytes to grow and expand from tumor tissue.
3. Adding interleukin-2 (IL-2) for cultivation: After adding a high concentration of IL-2, on the one hand, it provides survival signals for existing TIL cells, and on the other hand, stimulates larger-scale proliferation.
4. Conducting tumor-specific recognition testing: By conducting tumor-specific recognition testing on TIL cells, screening for TIL preparation processes that can more specifically kill tumors to ensure treatment effectiveness.
5. Reinfusion of expanded TIL cells for treatment: Reinfusing expanded and screened TIL cells into the patient’s body to achieve the treatment goal.
💪Characteristics of TIL therapy:
1. Rich in tumor-specific targets: TIL cells naturally infiltrate tumor sites and naturally have TCR clones capable of recognizing multiple tumor-specific antigens. Therefore, after cultivation and expansion, they can recognize and target multiple tumor antigens, thereby overcoming tumor heterogeneity and achieving broad-spectrum killing of cancer cells.
2. Good tumor tropism and strong infiltration ability: TIL cells have successfully infiltrated tumor tissue before and have a chemokine expression profile more related to peripheral blood T cells. Therefore, after reinfusion into the body, they will be attracted by tumor-related chemokines to better reach and infiltrate tumor tissue.
3. High safety and low cytotoxicity: TIL cells are immune cells already present in human tumor tissue and have undergone screening during early thymic development. Therefore, after reinfusion, they will not exert cytotoxic effects on other cells in the human body, demonstrating high safety. No major side effects have been observed since the development of TIL therapy.
🌟Therefore, TIL therapy is considered one of the most competitive and industrialized potential immunocyte therapy methods in the field of solid tumors. The approval of the world’s first TIL therapy for solid tumor treatment once again confirms this.
“If you’d like to inquire about the latest cancer-fighting technologies and treatments, you can contact us.”
whatsapp: 137 1795 9070
#TILTherapy #CancerTreatment #Immunotherapy #MedicalInnovation ##tils
